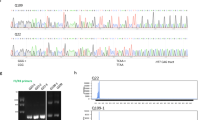
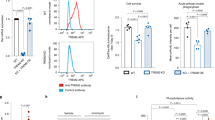
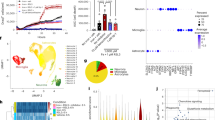
Abstract
Huntington's disease (HD) is a fatal neurodegenerative disorder caused by an extended polyglutamine repeat in the N terminus of the Huntingtin protein (HTT). Reactive microglia and elevated cytokine levels are observed in the brains of HD patients, but the extent to which neuroinflammation results from extrinsic or cell-autonomous mechanisms in microglia is unknown. Using genome-wide approaches, we found that expression of mutant Huntingtin (mHTT) in microglia promoted cell-autonomous pro-inflammatory transcriptional activation by increasing the expression and transcriptional activities of the myeloid lineage-determining factors PU.1 and C/EBPs. We observed elevated levels of PU.1 and its target genes in the brains of mouse models and individuals with HD. Moreover, mHTT-expressing microglia exhibited an increased capacity to induce neuronal death ex vivo and in vivo in the presence of sterile inflammation. These findings suggest a cell-autonomous basis for enhanced microglia reactivity that may influence non-cell-autonomous HD pathogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Streit, W.J., Walter, S.A. & Pennell, N.A. Reactive microgliosis. Prog. Neurobiol. 57, 563–581 (1999).
Samii, A., Nutt, J.G. & Ransom, B.R. Parkinson's disease. Lancet 363, 1783–1793 (2004).
Schellenberg, G.D. & Montine, T.J. The genetics and neuropathology of Alzheimer's disease. Acta Neuropathol. 124, 305–323 (2012).
The Huntington's Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell 72, 971–983 (1993).
Reiner, A. et al. Differential loss of striatal projection neurons in Huntington disease. Proc. Natl. Acad. Sci. USA 85, 5733–5737 (1988).
Ferrante, R.J. et al. Heterogeneous topographic and cellular distribution of huntingtin expression in the normal human neostriatum. J. Neurosci. 17, 3052–3063 (1997).
Trottier, Y. et al. Cellular localization of the Huntington's disease protein and discrimination of the normal and mutated form. Nat. Genet. 10, 104–110 (1995).
Lawson, L.J., Perry, V.H., Dri, P. & Gordon, S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 39, 151–170 (1990).
Kierdorf, K. et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci. 16, 273–280 (2013).
Tai, Y.F. et al. Microglial activation in presymptomatic Huntington's disease gene carriers. Brain 130, 1759–1766 (2007).
Sapp, E. et al. Early and progressive accumulation of reactive microglia in the Huntington disease brain. J. Neuropathol. Exp. Neurol. 60, 161–172 (2001).
Pavese, N. et al. Microglial activation correlates with severity in Huntington disease: a clinical and PET study. Neurology 66, 1638–1643 (2006).
Politis, M. et al. Microglial activation in regions related to cognitive function predicts disease onset in Huntington's disease: a multimodal imaging study. Hum. Brain Mapp. 32, 258–270 (2011).
Björkqvist, M. et al. A novel pathogenic pathway of immune activation detectable before clinical onset in Huntington's disease. J. Exp. Med. 205, 1869–1877 (2008).
Crocker, S.F., Costain, W.J. & Robertson, H.A. DNA microarray analysis of striatal gene expression in symptomatic transgenic Huntington's mice (R6/2) reveals neuroinflammation and insulin associations. Brain Res. 1088, 176–186 (2006).
Franciosi, S. et al. Age-dependent neurovascular abnormalities and altered microglial morphology in the YAC128 mouse model of Huntington disease. Neurobiol. Dis. 45, 438–449 (2012).
Silvestroni, A., Faull, R.L., Strand, A.D. & Moller, T. Distinct neuroinflammatory profile in post-mortem human Huntington's disease. Neuroreport 20, 1098–1103 (2009).
van Gool, W.A., van de Beek, D. & Eikelenboom, P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet 375, 773–775 (2010).
Giorgini, F., Guidetti, P., Nguyen, Q., Bennett, S.C. & Muchowski, P.J. A genomic screen in yeast implicates kynurenine 3-monooxygenase as a therapeutic target for Huntington disease. Nat. Genet. 37, 526–531 (2005).
Khoshnan, A. et al. Activation of the IkappaB kinase complex and nuclear factor-kappaB contributes to mutant huntingtin neurotoxicity. J. Neurosci. 24, 7999–8008 (2004).
Palazuelos, J. et al. Microglial CB2 cannabinoid receptors are neuroprotective in Huntington's disease excitotoxicity. Brain 132, 3152–3164 (2009).
Díaz-Hernández, M. et al. Altered P2X7-receptor level and function in mouse models of Huntington's disease and therapeutic efficacy of antagonist administration. FASEB J. 23, 1893–1906 (2009).
Medzhitov, R. Inflammation 2010: new adventures of an old flame. Cell 140, 771–776 (2010).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Bonifer, C., Hoogenkamp, M., Krysinska, H. & Tagoh, H. How transcription factors program chromatin–lessons from studies of the regulation of myeloid-specific genes. Semin. Immunol. 20, 257–263 (2008).
Walsh, J.C. et al. Cooperative and antagonistic interplay between PU.1 and GATA-2 in the specification of myeloid cell fates. Immunity 17, 665–676 (2002).
Chepelev, I., Wei, G., Wangsa, D., Tang, Q. & Zhao, K. Characterization of genome-wide enhancer-promoter interactions reveals co-expression of interacting genes and modes of higher order chromatin organization. Cell Res. 22, 490–503 (2012).
Brykczynska, U. et al. Repressive and active histone methylation mark distinct promoters in human and mouse spermatozoa. Nat. Struct. Mol. Biol. 17, 679–687 (2010).
Regha, K. et al. Active and repressive chromatin are interspersed without spreading in an imprinted gene cluster in the mammalian genome. Mol. Cell 27, 353–366 (2007).
Ghisletti, S. et al. Identification and characterization of enhancers controlling the inflammatory gene expression program in macrophages. Immunity 32, 317–328 (2010).
Tsukada, J., Yoshida, Y., Kominato, Y. & Auron, P.E. The CCAAT/enhancer (C/EBP) family of basic-leucine zipper (bZIP) transcription factors is a multifaceted highly-regulated system for gene regulation. Cytokine 54, 6–19 (2011).
Huber, R., Pietsch, D., Panterodt, T. & Brand, K. Regulation of C/EBPbeta and resulting functions in cells of the monocytic lineage. Cell. Signal. 24, 1287–1296 (2012).
Mangiarini, L. et al. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell 87, 493–506 (1996).
Heikkinen, T. et al. Characterization of neurophysiological and behavioral changes, MRI brain volumetry and 1H MRS in zQ175 knock-in mouse model of Huntington's disease. PLoS ONE 7, e50717 (2012).
Ginhoux, F. et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845 (2010).
Schulz, C. et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336, 86–90 (2012).
Wong, P.C. et al. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron 14, 1105–1116 (1995).
Boillée, S., Vande Velde, C. & Cleveland, D.W. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron 52, 39–59 (2006).
Boillée, S. et al. Onset and progression in inherited ALS determined by motor neurons and microglia. Science 312, 1389–1392 (2006).
Lehnardt, S. et al. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4–dependent pathway. Proc. Natl. Acad. Sci. USA 100, 8514–8519 (2003).
Saijo, K. et al. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell 137, 47–59 (2009).
Menalled, L.B. et al. Comprehensive behavioral and molecular characterization of a new knock-in mouse model of Huntington's Disease: zQ175. PLoS ONE 7, e49838 (2012).
Gu, X. et al. Pathological cell-cell interactions elicited by a neuropathogenic form of mutant Huntingtin contribute to cortical pathogenesis in HD mice. Neuron 46, 433–444 (2005).
Kraft, A.D., Kaltenbach, L.S., Lo, D.C. & Harry, G.J. Activated microglia proliferate at neurites of mutant huntingtin-expressing neurons. Neurobiol. Aging 33, 17–33 (2012).
Björkqvist, M. et al. A novel pathogenic pathway of immune activation detectable before clinical onset in Huntington's disease. J. Exp. Med. 205, 1869–1877 (2008).
Battista, D., Ferrari, C.C., Gage, F.H. & Pitossi, F.J. Neurogenic niche modulation by activated microglia: transforming growth factor beta increases neurogenesis in the adult dentate gyrus. Eur. J. Neurosci. 23, 83–93 (2006).
Monje, M.L., Toda, H. & Palmer, T.D. Inflammatory blockade restores adult hippocampal neurogenesis. Science 302, 1760–1765 (2003).
Ransome, M.I. & Hannan, A.J. Impaired basal and running-induced hippocampal neurogenesis coincides with reduced Akt signaling in adult R6/1 HD mice. Mol. Cell. Neurosci. 54, 93–107 (2013).
Simpson, J.M. et al. Altered adult hippocampal neurogenesis in the YAC128 transgenic mouse model of Huntington disease. Neurobiol. Dis. 41, 249–260 (2011).
Kohl, Z. et al. Impaired adult olfactory bulb neurogenesis in the R6/2 mouse model of Huntington's disease. BMC Neurosci. References for On-line Methods. 11, 114 (2010).
Gaspard, N. et al. Generation of cortical neurons from mouse embryonic stem cells. Nat. Protoc. 4, 1454–1463 (2009).
Marchetto, M.C. et al. Non-cell-autonomous effect of human SOD1 G37R astrocytes on motor neurons derived from human embryonic stem cells. Cell Stem Cell 3, 649–657 (2008).
Wang, X. & Seed, B. A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Res. 31, e154 (2003).
Marullo, M. et al. Expressed Alu repeats as a novel, reliable tool for normalization of real-time quantitative RT-PCR data. Genome Biol. 11, R9 (2010).
Ingolia, N.T., Ghaemmaghami, S., Newman, J.R. & Weissman, J.S. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science 324, 218–223 (2009).
Acknowledgements
We thank E. Mejia for Fluoro-Jade B staining, H. Kordasiewicz (ISIS Pharmaceuticals) for providing R6/2 mice, M. McAlonis, J. Artates and J. Boubaker for stereotaxic injection, University of California at San Diego (UCSD) Histology Core for PU.1 staining, the UCSD Human Embryonic Stem Cell Core Facility at Sanford Consortium for Regenerative Medicine for assistance with cell sorting, J. Corey-Bloom (Shiley-Marcos Alzheimer's Disease Research Center) for providing blood samples from HD patients, M. Hayden (University of British Columbia) for providing HTT and mHTT N548 aa original cDNA, S. Georges for assistance in quantification of TUNNEL assay, the Harvard Brain Tissue Resource Center (supported in part by PHS R24 MH068855), New York Brain Bank at Columbia University and Massachusetts General Hospital for HD post-mortem samples. C.L.T. and D.W.C. are supported by the CHDI Foundation. These studies were supported by US National Institutes of Health grants DK091183, DK063491, GM 069338 and CA17390 to C.K.G.
Author information
Authors and Affiliations
Contributions
C.K.G. and A.C. developed the study, conceived the experimental plans and analyzed the data. C.B. analyzed the genome-wide data. A.C. performed most of the biological, biochemical and molecular experiments. B.E.K. conceived and performed the TUNNEL assay experiment. D.G. performed microglia purification from adult mice brains. C.L.-T. provided Hdh175/175 SOD1G37R tissues, mice and performed the in vivo experiment. E.C. and C.Z. participated in the elaboration of the project and provided original constructs and mRNA from post-mortem human samples. D.W.C. and F.H.G. participated in experimental design and provided essential resources and reagents. C.K.G. and A.C. interpreted the data and wrote the manuscript. All of the authors read and edited the manuscript. C.K.G. supervised the entire work, directed the strategies, provided financial support and gave final approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 PU.1 and PU.1-C/EBPs target genes are upregulated in primary microglia but not in BMDM from R6/2 mice.
qRT-PCR analysis for Sfpi1 (a), Il6 (b) and Tnfα (c) mRNAs expression in primary microglia (mean±sd, n= 6 biological replicates, two-tailed paired student't test) and bone marrow derived macrophages (mean±sd, n= 5 biological replicates, two-tailed paired student't test) purified from non-transgenic littemates and R6/2 mice.
Supplementary Figure 2 Effect of siRNA knockdown of PU.1, C/EBPα and C/EBPβ.
Efficiency of siRNA knockdown in primary microglia derived from R6/2 mice and nontransgenic littermates as determined by qRT-PCR (mean±sd, n= 3 biological replicates, p values determined by two-tailed paired student t-test).
Supplementary Figure 3 Differential gene expression between wild-type microglia and wild-type macrophages.
Scatter Plot representing the differential gene expression observed in microglia from wild-type Hdh7/7 versus BMDM from wild-type Hdh7/7 mice.
Supplementary Figure 4 PU.1 and PU.1-C/EBPs target genes are upregulated in the striatum from R6/2 mice.
qRT-PCR analysis for Sfpi1 (a), Il6 (b), Tnfα (c), Irf1 (d) and Tlr2 (e) mRNAs expression in striatum from nontransgenic littermates, pre-symptomatic (5 weeks-old) and symptomatic (10 weeks-old) R6/2 mice. Each dot is representative of one mouse (unpaired student's test).
Supplementary Figure 5 PU.1 and PU.1-C/EBPs target genes are not differentially expressed in SOD1G37R mouse model of ALS.
qRT-PCR analysis for Sfpi1 (a), Il6 (b), Tnfα (c), Irf1 (d) and Tlr2 (e) mRNAs expression in striatum, cortex and spinal cord from nontransgenic littermates and SOD1G37R mice (8-12 months old). Each dot is representative of one mouse (unpaired student's test).
Supplementary Figure 6 Inflammation in vivo in HD individuals.
qRT-PCR analysis for IRF1 (a) and TNFα (b) mRNAs expression in striatum (first column, n= 9 individual per group), cortex (second column, n= 9 individual per group) and monocytes (third column, n= 5 individual per group) from controls and HD individuals. Each dot is representative of one individual. All p values were determined by unpaired student t-test. (c) IHC controls: brain section in presence of rabbit IgG (negative control) (i); spleen section in presence of rabbit IgG (negative control) (ii); spleen section in presence of diluting buffer (BSA, 1% bovine serum albumin in PBS phosphate buffered saline) (iii); H&E staining on spleen section (iv): PU.1 IHC staining on spleen section (positive control) (v); Von Willebrand factor IHC staining on spleen section (positive control for endothelial cells/blood vessels) (vi). Scale bar: 100μm.
Supplementary Figure 7 A model for mechanisms by which mutant Huntingtin influences the selection and activation of microglia enhancers.
Left: PU.1 and C/EBPs function in a collaborative manner to select microglia enhancers from inactive chromatin in basal conditions. Pro-inflammatory signals that activate transcription factors such as the p65 component of NFκB lead to inflammatory response. Right: mutant Huntingtin expression enhances this process by increasing PU.1 expression and PU.1-C/EBPs promoter binding, leading to increased enhancer activity under basal conditions that results in increased basal pro-inflammatory and neurotoxic genes expression. This phenomenon increases the sensitivity to pro-inflammatory signals. In fact, under conditions of sterile inflammation mutant Huntingtin-expressing microglia appears to be more efficient in inducing neuronal death.
Supplementary Figure 8 Full-length pictures of the blots presented in the main figures.
To examine proteins of interest on the same samples, blots were cut first and then probed with indicated antibodies.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 25393 kb)
Supplementary Table 1
Complete gene list for BV2 EV-, HTT N548-and mHTT N548-expressing cell lines. (XLSX 300 kb)
Supplementary Table 2
Complete gene list for microglia and BMDM from Hdh7/7 and Hdh175/175 mice. (XLSX 5194 kb)
Rights and permissions
About this article
Cite this article
Crotti, A., Benner, C., Kerman, B. et al. Mutant Huntingtin promotes autonomous microglia activation via myeloid lineage-determining factors. Nat Neurosci 17, 513–521 (2014). https://doi.org/10.1038/nn.3668
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3668