Abstract
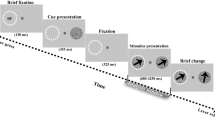
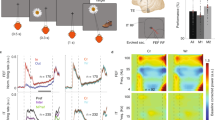
Short-term memory requires communication between multiple brain regions that collectively mediate the encoding and maintenance of sensory information. It has been suggested that oscillatory synchronization underlies intercortical communication. Yet, whether and how distant cortical areas cooperate during visual memory remains elusive. We examined neural interactions between visual area V4 and the lateral prefrontal cortex using simultaneous local field potential (LFP) recordings and single-unit activity (SUA) in monkeys performing a visual short-term memory task. During the memory period, we observed enhanced between-area phase synchronization in theta frequencies (3–9 Hz) of LFPs together with elevated phase locking of SUA to theta oscillations across regions. In addition, we found that the strength of intercortical locking was predictive of the animals' behavioral performance. This suggests that theta-band synchronization coordinates action potential communication between V4 and prefrontal cortex that may contribute to the maintenance of visual short-term memories.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Buzsáki, G. & Draguhn, A. Neuronal oscillations in cortical networks. Science 304, 1926–1929 (2004).
Fries, P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn. Sci. 9, 474–480 (2005).
Buzsáki, G. The hippocampo-neocortical dialogue. Cereb. Cortex 6, 81–92 (1996).
Fell, J. & Axmacher, N. The role of phase synchronization in memory processes. Nat. Rev. Neurosci. 12, 105–118 (2011).
Jones, M.W. & Wilson, M.A. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 3, e402 (2005).
O'Keefe, J. Hippocampus, theta, and spatial memory. Curr. Opin. Neurobiol. 3, 917–924 (1993).
Buzsáki, G. Theta oscillations in the hippocampus. Neuron 33, 325–340 (2002).
Kahana, M.J. et al. Human theta oscillations exhibit task dependence during virtual maze navigation. Nature 399, 781–784 (1999).
Raghavachari, S. et al. Gating of human theta oscillations by a working memory task. J. Neurosci. 21, 3175–3183 (2001).
Tesche, C.D. & Karhu, J. Theta oscillations index human hippocampal activation during a working memory task. Proc. Natl. Acad. Sci. USA 97, 919–924 (2000).
Rutishauser, U. et al. Human memory strength is predicted by theta-frequency phase-locking of single neurons. Nature 464, 903–907 (2010).
Sarnthein, J. et al. Synchronization between prefrontal and posterior association cortex during human working memory. Proc. Natl. Acad. Sci. USA 95, 7092–7096 (1998).
Orban, G.A. Higher order visual processing in macaque extrastriate cortex. Physiol. Rev. 88, 59–89 (2008).
Pasupathy, A. Neural basis of shape representation in the primate brain. Prog. Brain Res. 154, 293–313 (2006).
Zeki, S. The representation of colours in the cerebral cortex. Nature 284, 412–418 (1980).
Liebe, S., Logothetis, N.K. & Rainer, G. Dissociable effects of natural image structure and color on LFP and spiking activity in the lateral prefrontal cortex and extrastriate visual area V4. J. Neurosci. 31, 10215–10227 (2011).
Fries, P. et al. Modulation of oscillatory neuronal synchronization by selective visual attention. Science 291, 1560–1563 (2001).
Miller, E.K. & Cohen, J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 24, 167–202 (2001).
Fuster, J.M. & Alexander, G. Neuron activity related to short-term memory. Science 173, 652 (1971).
Pasternak, T. & Greenlee, M.W. Working memory in primate sensory systems. Nat. Rev. Neurosci. 6, 97–107 (2005).
Pesaran, B. et al. Temporal structure in neuronal activity during working memory in macaque parietal cortex. Nat. Neurosci. 5, 805–811 (2002).
Hoerzer, G.M. et al. Directed coupling in local field potentials of macaque v4 during visual short-term memory revealed by multivariate autoregressive models. Front. Comput. Neurosci. 4, 14 (2010).
Lee, H. et al. Phase locking of single neuron activity to theta oscillations during working memory in monkey extrastriate visual cortex. Neuron 45, 147–156 (2005).
Siegel, M., Warden, M.R. & Miller, E.K. Phase-dependent neuronal coding of objects in short-term memory. Proc. Natl. Acad. Sci. USA 106, 21341–21346 (2009).
Liebe, S. et al. Color and shape interactions in the recognition of natural scenes by human and monkey observers. J. Vis. 9, 14 (2009).
Lachaux, J.P. et al. Measuring phase synchrony in brain signals. Hum. Brain Mapp. 8, 194–208 (1999).
Azouz, R. & Gray, C.M. Adaptive coincidence detection and dynamic gain control in visual cortical neurons in vivo. Neuron 37, 513–523 (2003).
Rainer, G. & Miller, E.K. Timecourse of object-related neural activity in the primate prefrontal cortex during a short-term memory task. Eur. J. Neurosci. 15, 1244–1254 (2002).
Schack, B. et al. Phase-coupling of theta-gamma EEG rhythms during short-term memory processing. Int. J. Psychophysiol. 44, 143–163 (2002).
Weiss, S., Muller, H.M. & Rappelsberger, P. Theta synchronization predicts efficient memory encoding of concrete and abstract nouns. Neuroreport 11, 2357–2361 (2000).
Klimesch, W. Memory processes, brain oscillations and EEG synchronization. Int. J. Psychophysiol. 24, 61–100 (1996).
Tiesinga, P., Fellous, J.M. & Sejnowski, T.J. Regulation of spike timing in visual cortical circuits. Nat. Rev. Neurosci. 9, 97–107 (2008).
Volgushev, M., Chistiakova, M. & Singer, W. Modification of discharge patterns of neocortical neurons by induced oscillations of the membrane potential. Neuroscience 83, 15–25 (1998).
Womelsdorf, T. et al. Modulation of neuronal interactions through neuronal synchronization. Science 316, 1609–1612 (2007).
Fuentemilla, L. et al. Theta-coupled periodic replay in working memory. Curr. Biol. 20, 606–612 (2010).
Fell, J. et al. Phase-locking within human mediotemporal lobe predicts memory formation. Neuroimage 43, 410–419 (2008).
Guderian, S. & Duzel, E. Induced theta oscillations mediate large-scale synchrony with mediotemporal areas during recollection in humans. Hippocampus 15, 901–912 (2005).
Pavlides, C. et al. Long-term potentiation in the dentate gyrus is induced preferentially on the positive phase of theta-rhythm. Brain Res. 439, 383–387 (1988).
Huerta, P.T. & Lisman, J.E. Bidirectional synaptic plasticity induced by a single burst during cholinergic theta oscillation in Ca1 in vitro. Neuron 15, 1053–1063 (1995).
Lynch, M.A. Long-term potentiation and memory. Physiol. Rev. 84, 87–136 (2004).
Shadlen, M.N. & Newsome, W.T. Neural basis of a perceptual decision i the parietal cortex (area LIP) of the rhesus monkey. J. Neurophysiol. 86, 1916–1936 (2001).
Jensen, O. & Lisman, J.E. Hippocampal sequence-encoding driven by a cortical multi-item working memory buffer. Trends Neurosci. 28, 67–72 (2005).
Mongillo, G., Barak, O. & Tsodyks, M. Synaptic theory of working memory. Science 319, 1543–1546 (2008).
Mehta, M.R., Quirk, M.C. & Wilson, M.A. Experience-dependent asymmetric shape of hippocampal receptive fields. Neuron 25, 707–715 (2000).
Akam, T. & Kullmann, D.M. Oscillations and filtering networks support flexible routing of information. Neuron 67, 308–320 (2010).
Towe, A.L. & Harding, G.W. Extracellular microelectrode sampling bias. Exp. Neurol. 29, 366–381 (1970).
Graimann, B. & Pfurtscheller, G. Quantification and visualization of event-related changes in oscillatory brain activity in the time-frequency domain. Prog. Brain Res. 159, 79–97 (2006).
Tallon-Baudry, C. & Bertrand, O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn. Sci. 3, 151–162 (1999).
Siapas, A.G., Lubenov, E.V. & Wilson, M.A. Prefrontal phase locking to hippocampal theta oscillations. Neuron 46, 141–151 (2005).
Acknowledgements
We would like to thank J. Macke for discussions regarding analyses, and T. Mrsic-Flogel and J. Macke for useful comments on the manuscript. We also thank A.-L. Keller for help with histology. S.L. was supported by the Deutsche Forschungsgemeinschaft Sonderforschungsbereich 550 and a doctoral Fellowship of the Max Planck Society. G.M.H. was supported by projects FP7-231267 (“Self-Organized Recurrent Neural Learning for Language Processing”, ORGANIC) and FP7-506778 (Pattern Analysis, Statistical Modeling and Computational Learning, PASCAL2 ) of the European Union. G.R. is a European Science Foundation European Young Investigator.
Author information
Authors and Affiliations
Contributions
S.L. and G.R. designed the experiments. S.L. conducted the experiments. S.L. and G.M.H. analyzed the data. S.L. wrote the manuscript with contributions from G.M.H. and G.R. N.K.L. and G.R. supervised the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 (PDF 3841 kb)
Rights and permissions
About this article
Cite this article
Liebe, S., Hoerzer, G., Logothetis, N. et al. Theta coupling between V4 and prefrontal cortex predicts visual short-term memory performance. Nat Neurosci 15, 456–462 (2012). https://doi.org/10.1038/nn.3038
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3038
This article is cited by
-
Learning how network structure shapes decision-making for bio-inspired computing
Nature Communications (2023)
-
Modulation of Neuronal Activity and Saccades at Theta Rhythm During Visual Search in Non-human Primates
Neuroscience Bulletin (2022)
-
Sensory representation of visual stimuli in the coupling of low-frequency phase to spike times
Brain Structure and Function (2022)
-
Mechanisms of Functioning of the Connectome Including the Neocortex, Hippocampus, Basal Ganglia, Cerebellum, and Thalamus
Neuroscience and Behavioral Physiology (2022)
-
The olfactory bulb modulates entorhinal cortex oscillations during spatial working memory
The Journal of Physiological Sciences (2021)