Abstract
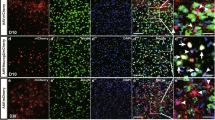
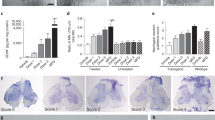
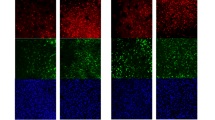
Cellular abnormalities in amyotrophic lateral sclerosis (ALS) are not limited to motor neurons. Astrocyte dysfunction also occurs in human ALS and transgenic rodents expressing mutant human SOD1 protein (SOD1G93A). Here we investigated focal enrichment of normal astrocytes using transplantation of lineage-restricted astrocyte precursors, called glial-restricted precursors (GRPs). We transplanted GRPs around cervical spinal cord respiratory motor neuron pools, the principal cells whose dysfunction precipitates death in ALS. GRPs survived in diseased tissue, differentiated efficiently into astrocytes and reduced microgliosis in the cervical spinal cords of SOD1G93A rats. GRPs also extended survival and disease duration, attenuated motor neuron loss and slowed declines in forelimb motor and respiratory physiological functions. Neuroprotection was mediated in part by the primary astrocyte glutamate transporter GLT1. These findings indicate the feasibility and efficacy of transplantation-based astrocyte replacement and show that targeted multisegmental cell delivery to the cervical spinal cord is a promising therapeutic strategy for slowing focal motor neuron loss associated with ALS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bruijn, L.I., Miller, T.M. & Cleveland, D.W. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu. Rev. Neurosci. 27, 723–749 (2004).
Rosen, D.R. et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362, 59–62 (1993).
Bruijn, L.I. et al. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron 18, 327–338 (1997).
Gurney, M.E. et al. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science 264, 1772–1775 (1994).
Wong, P.C. et al. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron 14, 1105–1116 (1995).
Howland, D.S. et al. Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant–mediated amyotrophic lateral sclerosis (ALS). Proc. Natl. Acad. Sci. USA 99, 1604–1609 (2002).
Boillee, S. et al. Onset and progression in inherited ALS determined by motor neurons and microglia. Science 312, 1389–1392 (2006).
Clement, A.M. et al. Wild-type non-neuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science 302, 113–117 (2003).
Di Giorgio, F.P., Carrasco, M.A., Siao, M.C., Maniatis, T. & Eggan, K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell–based ALS model. Nat. Neurosci. 10, 608–614 (2007).
Lino, M.M., Schneider, C. & Caroni, P. Accumulation of SOD1 mutants in postnatal motoneurons does not cause motoneuron pathology or motoneuron disease. J. Neurosci. 22, 4825–4832 (2002).
Nagai, M. et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat. Neurosci. 10, 615–622 (2007).
Pramatarova, A., Laganiere, J., Roussel, J., Brisebois, K. & Rouleau, G.A. Neuron-specific expression of mutant superoxide dismutase 1 in transgenic mice does not lead to motor impairment. J. Neurosci. 21, 3369–3374 (2001).
Yamanaka, K. et al. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat. Neurosci. 11, 251–253 (2008).
Pekny, M. & Nilsson, M. Astrocyte activation and reactive gliosis. Glia 50, 427–434 (2005).
Rothstein, J.D. et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16, 675–686 (1996).
Rothstein, J.D. et al. Localization of neuronal and glial glutamate transporters. Neuron 13, 713–725 (1994).
Maragakis, N.J. & Rothstein, J.D. Mechanisms of disease: astrocytes in neurodegenerative disease. Nat. Clin. Pract. Neurol. 2, 679–689 (2006).
Rothstein, J.D., Van Kammen, M., Levey, A.I., Martin, L.J. & Kuncl, R.W. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann. Neurol. 38, 73–84 (1995).
Rao, M.S. & Mayer-Proschel, M. Glial-restricted precursors are derived from multipotent neuroepithelial stem cells. Dev. Biol. 188, 48–63 (1997).
Rao, M.S., Noble, M. & Mayer-Proschel, M. A tripotential glial precursor cell is present in the developing spinal cord. Proc. Natl. Acad. Sci. USA 95, 3996–4001 (1998).
Llado, J. et al. Degeneration of respiratory motor neurons in the SOD1 G93A transgenic rat model of ALS. Neurobiol. Dis. 21, 110–118 (2006).
Tankersley, C.G., Haenggeli, C. & Rothstein, J.D. Respiratory impairment in a mouse model of amyotrophic lateral sclerosis. J. Appl. Physiol. 102, 926–932 (2007).
Matsumoto, A. et al. Disease progression of human SOD1 (G93A) transgenic ALS model rats. J. Neurosci. Res. 83, 119–133 (2006).
Maragakis, N.J. et al. Glial restricted precursors protect against chronic glutamate neurotoxicity of motor neurons in vitro. Glia 50, 145–159 (2005).
Mitsui, T., Fischer, I., Shumsky, J.S. & Murray, M. Transplants of fibroblasts expressing BDNF and NT-3 promote recovery of bladder and hindlimb function following spinal contusion injury in rats. Exp. Neurol. 194, 410–431 (2005).
Maragakis, N.J. & Rothstein, J.D. Glutamate transporters: animal models to neurologic disease. Neurobiol. Dis. 15, 461–473 (2004).
Federici, T. & Boulis, N.M. Gene-based treatment of motor neuron diseases. Muscle Nerve 33, 302–323 (2006).
Rothstein, J.D., Martin, L.J. & Kuncl, R.W. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. N. Engl. J. Med. 326, 1464–1468 (1992).
Rothstein, J.D. et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature 433, 73–77 (2005).
Kiaei, M., Kipiani, K., Chen, J., Calingasan, N.Y. & Beal, M.F. Peroxisome proliferator-activated receptor-gamma agonist extends survival in transgenic mouse model of amyotrophic lateral sclerosis. Exp. Neurol. 191, 331–336 (2005).
Sofroniew, M.V. Reactive astrocytes in neural repair and protection. Neuroscientist 11, 400–407 (2005).
Deshpande, D.M. et al. Recovery from paralysis in adult rats using embryonic stem cells. Ann. Neurol. 60, 32–44 (2006).
Harper, J.M. et al. Axonal growth of embryonic stem cell–derived motoneurons in vitro and in motoneuron-injured adult rats. Proc. Natl. Acad. Sci. USA 101, 7123–7128 (2004).
Kerr, D.A. et al. Human embryonic germ cell derivatives facilitate motor recovery of rats with diffuse motor neuron injury. J. Neurosci. 23, 5131–5140 (2003).
Wu, P. et al. Region-specific generation of cholinergic neurons from fetal human neural stem cells grafted in adult rats. Nat. Neurosci. 5, 1271–1278 (2002).
Aebischer, P. et al. Gene therapy for amyotrophic lateral sclerosis (ALS) using a polymer encapsulated xenogenic cell line engineered to secrete hCNTF. Hum. Gene Ther. 7, 851–860 (1996).
Aebischer, P. et al. Intrathecal delivery of CNTF using encapsulated genetically modified xenogeneic cells in amyotrophic lateral sclerosis patients. Nat. Med. 2, 696–699 (1996).
Corti, S. et al. Wild-type bone marrow cells ameliorate the phenotype of SOD1-G93A ALS mice and contribute to CNS, heart and skeletal muscle tissues. Brain 127, 2518–2532 (2004).
Corti, S. et al. Neural stem cells LewisX+ CXCR4+ modify disease progression in an amyotrophic lateral sclerosis model. Brain 130, 1289–1305 (2007).
Garbuzova-Davis, S. et al. Intraspinal implantation of hNT neurons into SOD1 mice with apparent motor deficit. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2, 175–180 (2001).
Garbuzova-Davis, S. et al. Positive effect of transplantation of hNT neurons (NTera 2/D1 cell line) in a model of familial amyotrophic lateral sclerosis. Exp. Neurol. 174, 169–180 (2002).
Garbuzova-Davis, S. et al. Intravenous administration of human umbilical cord blood cells in a mouse model of amyotrophic lateral sclerosis: distribution, migration and differentiation. J. Hematother. Stem Cell Res. 12, 255–270 (2003).
Hemendinger, R. et al. Sertoli cells improve survival of motor neurons in SOD1 transgenic mice, a model of amyotrophic lateral sclerosis. Exp. Neurol. 196, 235–243 (2005).
Llado, J., Haenggeli, C., Maragakis, N.J., Snyder, E.Y. & Rothstein, J.D. Neural stem cells protect against glutamate-induced excitotoxicity and promote survival of injured motor neurons through the secretion of neurotrophic factors. Mol. Cell. Neurosci. 27, 322–331 (2004).
Martin, L.J. & Liu, Z. Adult olfactory bulb neural precursor cell grafts provide temporary protection from motor neuron degeneration, improve motor function and extend survival in amyotrophic lateral sclerosis mice. J. Neuropathol. Exp. Neurol. 66, 1002–1018 (2007).
Xu, L. et al. Human neural stem cell grafts ameliorate motor neuron disease in SOD-1 transgenic rats. Transplantation 82, 865–875 (2006).
Yan, J. et al. Combined immunosuppressive agents or CD4 antibodies prolong survival of human neural stem cell grafts and improve disease outcomes in amyotrophic lateral sclerosis transgenic mice. Stem Cells 24, 1976–1985 (2006).
Klein, S.M. et al. GDNF delivery using human neural progenitor cells in a rat model of ALS. Hum. Gene Ther. 16, 509–521 (2005).
Suzuki, M. et al. GDNF secreting human neural progenitor cells protect dying motor neurons, but not their projection to muscle, in a rat model of familial ALS. PLoS ONE 2, e689 (2007).
Acknowledgements
We thank all members of the Maragakis and Rothstein labs for helpful discussion and K. Tanaka for providing GLT1-null mice. This work was supported by the Robert Packard Center for ALS Research (to N.J.M.), the ALS Association (to N.J.M.) and the National Institutes of Health (F32-NS059155 to A.C.L., R01-NS33958 to J.D.R. and R01-NS41680 to J.D.R. and N.J.M.).
Author information
Authors and Affiliations
Contributions
A.C.L. designed and conducted the experiments, analyzed the data, prepared the figures and wrote the manuscript. N.J.M. supervised the project and participated in designing experiments and writing the manuscript. M.S.R. and J.D.R. participated in designing experiments and writing the manuscript. B.R., A.C.P. and C.D. conducted experiments.
Corresponding author
Ethics declarations
Competing interests
M.S.R. works for Invitrogen, which is a supplier of reagents to the stem cell research communuty. M.S.R. was a scientific founder of Q Therapeutics, Inc. and is a non-paid scientific consultant. J.D.R. is a consultant to Ruxton Pharmaceuticals. N.J.M. is a non-paid scientific consultant to Q Therapeutics, Inc.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 and Supplementary Methods (PDF 296 kb)
Supplementary Text and Figures
Supplementary Video 1 (AVI 12288 kb)
Rights and permissions
About this article
Cite this article
Lepore, A., Rauck, B., Dejea, C. et al. Focal transplantation–based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat Neurosci 11, 1294–1301 (2008). https://doi.org/10.1038/nn.2210
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2210