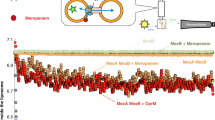
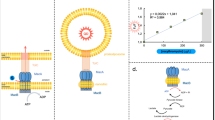
Abstract
The MacA–MacB–TolC assembly of Escherichia coli is a transmembrane machine that spans the cell envelope and actively extrudes substrates, including macrolide antibiotics and polypeptide virulence factors. These transport processes are energized by the ATPase MacB, a member of the ATP-binding cassette (ABC) superfamily. We present an electron cryo-microscopy structure of the ABC-type tripartite assembly at near-atomic resolution. A hexamer of the periplasmic protein MacA bridges between a TolC trimer in the outer membrane and a MacB dimer in the inner membrane, generating a quaternary structure with a central channel for substrate translocation. A gating ring found in MacA is proposed to act as a one-way valve in substrate transport. The MacB structure features an atypical transmembrane domain with a closely packed dimer interface and a periplasmic opening that is the likely portal for substrate entry from the periplasm, with subsequent displacement through an allosteric transport mechanism.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kobayashi, N., Nishino, K. & Yamaguchi, A. Novel macrolide-specific ABC-type efflux transporter in Escherichia coli. J. Bacteriol. 183, 5639–5644 (2001).
Vallet-Gely, I. et al. Association of hemolytic activity of Pseudomonas entomophila, a versatile soil bacterium, with cyclic lipopeptide production. Appl. Environ. Microbiol. 76, 910–921 (2010).
Cho, H. & Kang, H. The PseEF efflux system is a virulence factor of Pseudomonas syringae pv. syringae. J. Microbiol. 50, 79–90 (2012).
Turlin, E. et al. Protoporphyrin (PPIX) efflux by the MacAB–TolC pump in Escherichia coli. Microbiologyopen 3, 849–859 (2014).
Yamanaka, H., Kobayashi, H., Takahashi, E. & Okamoto, K. MacAB is involved in the secretion of Escherichia coli heat-stable enterotoxin II. J. Bacteriol. 190, 7693–7698 (2008).
Lu, S. & Zgurskaya, H. I. MacA, a periplasmic membrane fusion protein of the macrolide transporter MacAB–TolC, binds lipopolysaccharide core specifically and with high affinity. J. Bacteriol. 195, 4865–4872 (2013).
Lu, S. & Zgurskaya, H. I. Role of ATP binding and hydrolysis in assembly of MacAB–TolC macrolide transporter. Mol. Microbiol. 86, 1132–1143 (2012).
Tikhonova, E. B., Devroy, V. K., Lau, S. Y. & Zgurskaya, H. I. Reconstitution of the Escherichia coli macrolide transporter: the periplasmic membrane fusion protein MacA stimulates the ATPase activity of MacB. Mol. Microbiol. 63, 895–910 (2007).
Dawson, R. J. P. & Locher, K. P. Structure of a bacterial multidrug ABC transporter. Nature 443, 180–185 (2006).
Ward, A., Reyes, C. L., Yu, J., Roth, C. B. & Chang, G. Flexibility in the ABC transporter MsbA: alternating access with a twist. Proc. Natl Acad. Sci. USA 104, 19005–19010 (2007).
Gutmann, D. A. P., Ward, A., Urbatsch, I. L., Chang, G. & van Veen, H. W. Understanding polyspecificity of multidrug ABC transporters: closing in on the gaps in ABCB1. Trends Biochem. Sci. 35, 36–42 (2010).
Doshi, R., Woebking, B. & van Veen, H. W. Dissection of the conformational cycle of the multidrug/lipidA ABC exporter MsbA. Proteins 78, 2867–2872 (2010).
Choudhury, H. G. et al. Structure of an antibacterial peptide ATP-binding cassette transporter in a novel outward occluded state. Proc. Natl Acad. Sci. USA 111, 9145–9150 (2014).
Lee, J.-Y. et al. Crystal structure of the human sterol transporter ABCG5/ABCG8. Nature 533, 561–564 (2016).
Aittoniemi, J. et al. SUR1: a unique ATP-binding cassette protein that functions as an ion channel regulator. Philos. Trans. R. Soc. B 364, 257–267 (2009).
Locher, K. P. Mechanistic diversity in ATP-binding cassette (ABC) transporters. Nat. Struct. Mol. Biol. 23, 487–493 (2016).
Perez, C. et al. Structure and mechanism of an active lipid-linked oligosaccharide flippase. Nature 524, 433–438 (2015).
Khare, D., Oldham, M. L., Orelle, C., Davidson, A. L. & Chen, J. Alternating access in maltose transporter mediated by rigid-body rotations. Mol. Cell 33, 528–536 (2009).
Woo, J.-S., Zeltina, A., Goetz, B. A. & Locher, K. P. X-ray structure of the Yersinia pestis heme transporter HmuUV. Nat. Struct. Mol. Biol. 19, 1310–1315 (2012).
Korkhov, V. M., Mireku, S. A. & Locher, K. P. Structure of AMP-PNP-bound vitamin B12 transporter BtuCD-F. Nature 490, 367–372 (2012).
Song, S., Kim, J.-S., Lee, K. & Ha, N.-C. Molecular architecture of the bacterial tripartite multidrug efflux pump focusing on the adaptor bridging model. J. Microbiol. 53, 355–364 (2015).
Zgurskaya, H. I., Weeks, J. W., Ntreh, A. T., Nickels, L. M. & Wolloscheck, D. Mechanism of coupling drug transport reactions located in two different membranes. Front. Microbiol. 6, 100 (2015).
Du, D., van Veen, H. W. & Luisi, B. F. Assembly and operation of bacterial tripartite multidrug efflux pumps. Trends Microbiol. 23, 311–319 (2015).
Yamanaka, H., Izawa, H. & Okamoto, K. Carboxy-terminal region involved in activity of Escherichia coli TolC. J. Bacteriol. 183, 6961–6964 (2001).
Du, D. et al. Structure of the AcrAB–TolC multidrug efflux pump. Nature 509, 512–515 (2014).
Daury, L. et al. Tripartite assembly of RND multidrug efflux pumps. Nat. Commun. 7, 10731 (2016).
Kim, J.-S. et al. Structure of the tripartite multidrug efflux pump AcrAB–TolC suggests an alternative assembly mode. Mol. Cells 38, 180–186 (2015).
Lin, H. T. et al. Macb ABC transporter is a dimer whose ATPase activity and macrolide-binding capacity are regulated by the membrane fusion protein MacA. J. Biol. Chem. 284, 1145–1154 (2009).
Xu, Y. et al. Crystal structure of the periplasmic region of MacB, a noncanonic ABC transporter. Biochemistry 48, 5218–5225 (2009).
Yum, S. et al. Crystal structure of the periplasmic component of a tripartite macrolide-specific efflux pump. J. Mol. Biol. 387, 1286–1297 (2009).
Calladine, C. R., Sharff, A. & Luisi, B. How to untwist an α-helix: structural principles of an α-helical barrel. J. Mol. Biol. 305, 603–618 (2001).
Modali, S. D. & Zgurskaya, H. I. The periplasmic membrane proximal domain of MacA acts as a switch in stimulation of ATP hydrolysis by MacB transporter. Mol. Microbiol. 81, 937–951 (2011).
Bai, X., Rajendra, E., Yang, G., Shi, Y. & Scheres, S. H. W. Sampling the conformational space of the catalytic subunit of human γ-secretase. eLife 4, e11182 (2015).
Jeong, H. et al. Pseudoatomic structure of the tripartite multidrug efflux pump AcrAB–TolC reveals the intermeshing cogwheel-like interaction between AcrA and TolC. Structure 24, 272–276 (2016).
Xu, Y. et al. The tip region of the MacA α-hairpin is important for the binding to TolC to the Escherichia coli MacAB–TolC pump. Biochem. Biophys. Res. Commun. 394, 962–965 (2010).
Xu, Y. et al. Functional implications of an intermeshing cogwheel-like interaction between TolC and MacA in the action of macrolide-specific efflux pump MacAB–TolC. J. Biol. Chem. 286, 13541–13549 (2011).
Lee, M. et al. The α-barrel tip region of Escherichia coli TolC homologs of Vibrio vulnificus interacts with the MacA protein to form the functional macrolide-specific efflux pump MacAB–TolC. J. Microbiol. 51, 154–159 (2013).
Dong, C. et al. Wza the translocon for E. coli capsular polysaccharides defines a new class of membrane protein. Nature 444, 226–229 (2006).
Suloway, C. et al. Automated molecular microscopy: the new Leginon system. J. Struct. Biol. 151, 41–60 (2005).
Meyerson, J. R. et al. Self-assembled monolayers improve protein distribution on holey carbon cryo-EM supports. Sci. Rep. 4, 7084 (2014).
Russo, C. J. & Passmore, L. A. Electron microscopy: ultrastable gold substrates for electron cryomicroscopy. Science 346, 1377–1380 (2014).
Li, X. et al. Electron counting and beam-induced motion correction enable near-atomic-resolution single-particle cryo-EM. Nat. Methods 10, 584–590 (2013).
Zhang, K. Gctf: real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Scheres, S. H. W. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Scheres, S. H. W. Semi-automated selection of cryo-EM particles in RELION-1.3. J. Struct. Biol. 189, 114–122 (2015).
Tang, G. et al. EMAN2: an extensible image processing suite for electron microscopy. J. Struct. Biol. 157, 38–46 (2007).
Scheres, S. H. Beam-induced motion correction for sub-megadalton cryo-EM particles. eLife 3, e03665 (2014).
Scheres, S. H. W. Processing of structurally heterogeneous cryo-EM data in RELION. Methods Enzymol. 579, 125–157 (2016).
Scheres, S. H. W. & Chen, S. Prevention of overfitting in cryo-EM structure determination. Nat. Methods 9, 853–854 (2012).
Kucukelbir, A., Sigworth, F. J. & Tagare, H. D. Quantifying the local resolution of cryo-EM density maps. Nat. Methods 11, 63–65 (2014).
Abraham, M. J. et al. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1, 19–25 (2015).
Hornak, V. et al. Comparison of multiple AMBER force fields and development of improved protein backbone parameters. Proteins 65, 712–725 (2006).
Wang, J., Cieplak, P. & Kollman, P. A. How well does a restrained electrostatic potential (RESP) model perform in calculating conformational energies of organic and biological molecules? J. Comput. Chem. 21, 1049–1074 (2000).
Case, D. A. et al. AMBER 12 (Univ. California, 2012).
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926 (1983).
Joung, I. S. & Cheatham, T. E. Determination of alkali and halide monovalent ion parameters for use in explicitly solvated biomolecular simulations. J. Phys. Chem. B 112, 9020–9041 (2008).
Grubmüller, H., Heymann, B. & Tavan, P. Ligand binding: molecular mechanics calculation of the streptavidin–biotin rupture force. Science 271, 997–999 (1996).
Acknowledgements
This work was supported by the Wellcome Trust (to B.F.L.), the Human Frontiers Science Program (to B.F.L., H.W.v.V. and S.M.), a Marie Curie International Outgoing Fellowship (to A.W.P.F.), the UK Medical Research Council (MC_UP_A025_1013, to S.H.W.S.), a Wellcome Trust ISSF award (grant no. WT097818MF), the Scottish Universities’ Physics Alliance (to U.Z. and S.L.) and the MRC Mitochondrial Biology Unit (grant no. U105663141). A.N. is the recipient of a Herchel–Smith Scholarship. The authors thank J. Skepper for help using the EM microscope and the imaging facility at Cambridge University, H. Zhou for providing access to the electron microscope at the University of California at Los Angeles, J. Grimmett and T. Darling for support with high-performance computing at the MRC Laboratory of Molecular Biology, S. Rankin for help with computing on the High-Performance Computing System at the University of Cambridge, and L. Packman for mass spectrometry analyses. The authors thank the staff of the Diamond Light Source for access to the eBIC facility. The EM maps have been deposited in the Electron Microscopy Databank.
Author information
Authors and Affiliations
Contributions
D.D., B.F.L. and S.H.W.S. designed the project. D.D. purified the fusion and disulfide-linkage-stabilized MacAB–TolC complexes. D.D., A.W.P.F., X.C.B. and J.N.B. obtained and analysed the single-particle cryo-EM data. U.O. and S.M. built the homology model of MacB. D.D. and B.F.L. devised a model of MacAB–TolC based on the cryo-EM map. A.N. and H.W.v.V. conducted MIC assays on the MacAB–TolC pump. S.L. and U.Z. carried out molecular dynamics simulations of MacA. D.D., B.F.L. and S.H.W.S. wrote the paper. All authors contributed to editing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1–14. (PDF 5660 kb)
Rights and permissions
About this article
Cite this article
Fitzpatrick, A., Llabrés, S., Neuberger, A. et al. Structure of the MacAB–TolC ABC-type tripartite multidrug efflux pump. Nat Microbiol 2, 17070 (2017). https://doi.org/10.1038/nmicrobiol.2017.70
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nmicrobiol.2017.70