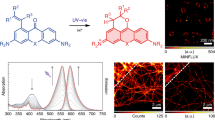
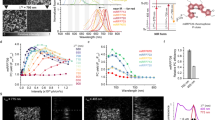
Abstract
We have developed a simple correlative photooxidation method that allows for the direct ultrastructural visualization of the green fluorescent protein (GFP) upon illumination. The method, termed GRAB for GFP recognition after bleaching, uses oxygen radicals generated during the GFP bleaching process to photooxidize 3,3′-diaminobenzidine (DAB) into an electron-dense precipitate that can be visualized by routine electron microscopy and electron tomography. The amount of DAB product produced by the GRAB method appears to be linear with the initial fluorescence, and the resulting images are of sufficient quality to reveal detailed spatial information. This is exemplified by the observed intra–Golgi stack and intracisternal distribution of a human Golgi resident glycosylation enzyme, N-acetylgalactosaminyltransferase-2 fused either to enhanced GFP or CFP.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
28 November 2005
Original SI Figures 1, 2, and 3 did not have figure legends in the PDF. New SI Figures 1, 2, and 3 with legends should replace the originals.
References
Maranto, A.R. Neuronal mapping: a photooxidation reaction makes Lucifer yellow useful for electron microscopy. Science 217, 953–955 (1982).
Gaietta, G. et al. Multicolor and electron microscopic imaging of connexin trafficking. Science 296, 503–507 (2002).
Egner, A. & Hell, S.W. Fluorescence microscopy with super-resolved optical sections. Trends Cell Biol. 15, 207–215 (2005).
Monosov, E.Z., Wenzel, T.J., Luers, G.H., Heyman, J.A. & Subramani, S. Labeling of peroxisomes with green fluorescent protein in living P. pastoris cells. J. Histochem. Cytochem. 44, 581–589 (1996).
Svitkina, T.M. & Borisy, G.G. Correlative light and electron microscopy of the cytoskeleton of cultured cells. Methods Enzymol. 298, 570–592 (1998).
Castejon, O.J. & Castejon, H.V. Correlative microscopy of cerebellar Golgi cells. Biocell 24, 13–30 (2000).
Mironov, A.A., Polishchuk, R.S. & Luini, A. Visualizing membrane traffic in vivo by combined video fluorescence and 3D electron microscopy. Trends Cell Biol. 10, 349–353 (2000).
Pagano, R.E., Sepanski, M.A. & Martin, O.C. Molecular trapping of a fluorescent ceramide analogue at the Golgi apparatus of fixed cells: interaction with endogenous lipids provides a trans-Golgi marker for both light and electron microscopy. J. Cell Biol. 109, 2067–2079 (1989).
Harata, N., Ryan, T.A., Smith, S.J., Buchanan, J. & Tsien, R.W. Visualizing recycling synaptic vesicles in hippocampal neurons by FM 1–43 photoconversion. Proc. Natl. Acad. Sci. USA 98, 12748–12753 (2001).
Marsh, B.J., Mastronarde, D.N., Buttle, K.F., Howell, K.E. & McIntosh, J.R. Organellar relationships in the Golgi region of the pancreatic beta cell line, HIT-T15, visualized by high resolution electron tomography. Proc. Natl. Acad. Sci. USA 98, 2399–2406 (2001).
Storrie, B. et al. Recycling of golgi-resident glycosyltransferases through the ER reveals a novel pathway and provides an explanation for nocodazole-induced Golgi scattering. J. Cell Biol. 143, 1505–1521 (1998).
Lubke, J. Photoconversion of diaminobenzidine with different fluorescent neuronal markers into a light and electron microscopic dense reaction product. Microsc. Res. Tech. 24, 2–14 (1993).
Nilsson, T. et al. Overlapping distribution of two glycosyltransferases in the Golgi apparatus of HeLa cells. J. Cell Biol. 120, 5–13 (1993).
Rabouille, C. et al. Mapping the distribution of Golgi enzymes involved in the construction of complex oligosaccharides. J. Cell Sci. 108, 1617–1627 (1995).
Rottger, S. et al. Localization of three human polypeptide GalNAc-transferases in HeLa cells suggests initiation of O-linked glycosylation throughout the Golgi apparatus. J. Cell Sci. 111, 45–60 (1998).
Orci, L., Perrelet, A. & Rothman, J.E. Vesicles on strings: morphological evidence for processive transport within the Golgi stack. Proc. Natl. Acad. Sci. USA 95, 2279–2283 (1998).
Shorter, J., Beard, M.B., Seemann, J., Dirac-Svejstrup, A.B. & Warren, G. Sequential tethering of Golgins and catalysis of SNAREpin assembly by the vesicle-tethering protein p115. J. Cell Biol. 157, 45–62 (2002).
Cosson, P., Amherdt, M., Rothman, J.E. & Orci, L. A resident Golgi protein is excluded from peri-Golgi vesicles in NRK cells. Proc. Natl. Acad. Sci. USA 99, 12831–12834 (2002).
Kweon, H.S. et al. Golgi enzymes are enriched in perforated zones of golgi cisternae but are depleted in COPI vesicles. Mol. Biol. Cell 15, 4710–4724 (2004).
Martinez-Menarguez, J.A. et al. Peri-Golgi vesicles contain retrograde but not anterograde proteins consistent with the cisternal progression model of intra-Golgi transport. J. Cell Biol. 155, 1213–1224 (2001).
Lanoix, J. et al. GTP hydrolysis by arf-1 mediates sorting and concentration of Golgi resident enzymes into functional COP I vesicles. EMBO J. 18, 4935–4948 (1999).
Lin, C.C., Love, H.D., Gushue, J.N., Bergeron, J.J. & Ostermann, J.E.R. Golgi intermediates acquire Golgi enzymes by brefeldin A–sensitive retrograde transport in vitro. J. Cell Biol. 147, 1457–1472 (1999).
Malsam, J., Satoh, A., Pelletier, L. & Warren, G. Golgin tethers define subpopulations of COPI vesicles. Science 307, 1095–1098 (2005).
McIntosh, R., Nicastro, D. & Mastronarde, D. New views of cells in 3D: an introduction to electron tomography. Trends Cell Biol. 15, 43–51 (2005).
Paabo, S., Weber, F., Nilsson, T., Schaffner, W. & Peterson, P.A. Structural and functional dissection of an MHC class I antigen-binding adenovirus glycoprotein. EMBO J. 5, 1921–1927 (1986).
Baschong, W., Suetterlin, R. & Laeng, R.H. Control of autofluorescence of archival formaldehyde-fixed, paraffin-embedded tissue in confocal laser scanning microscopy (CLSM). J. Histochem. Cytochem. 49, 1565–1572 (2001).
Hayat, M.A. Principles and techniques of electron microscopy. (Van Nostrand Reinhold Company, New York, 1970).
Ziese, U. et al. Automated high-throughput electron tomography by pre-calibration of image shifts. J. Microsc. 205, 187–200 (2002).
Kremer, J.R., Mastronarde, D.N. & McIntosh, J.R. Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 116, 71–76 (1996).
Acknowledgements
We wish to thank R. Pepperkok, J. Rietdorf and K. Miura (Advanced Light Microscopy Facility, EMBL Heidelberg) for discussions and help in image analysis, M. Lebbink (Utrecht, The Netherlands) for his help using Amira software, M. Axelsson for help in preparing mitotic populations of HeLa cells and Swegene for its support of the Center for Cellular Imaging in Gothenburg. HeLa cells stably expressing α-tubulin-EGFP were a generous gift of J. Lipp and J.-M. Peters (IMP Vienna, Austria). This work was supported by an EMBO fellowship (M.G.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
HeLa cells stably expressing GalNAc-T2CFP were cultivated for 22 hours in 100μM nocodazole, then fixed and processed for photooxidation. (PDF 771 kb)
Supplementary Fig. 2
HeLa cells were infected with a recombinant adenovirus strain expressing the temperature sensitive vesicular stomatitis virus (VSV) G protein derived from the VSV Orsay ts045 strain and fused to ECFP. (PDF 1417 kb)
Supplementary Fig. 3
Peri-Golgi vesicles in perpendicular projections. (PDF 1487 kb)
Supplementary Fig. 4
Microtubules. (PDF 1093 kb)
Supplementary Video 1
Tomography study of a part of the Golgi stack containing DAB-precipitate representing the Golgi-resident enzyme, GalNAc-T2EGFP. It starts with a ‘virtual flight through’ of the tomogram in z-axis direction. This is followed by the manual tracing of membranes to yield a 3D representation of the Golgi stack in different colors for each cisterna. Rotation of the final 3D representation shows GFP-containing cisternae in green with a highlight of the peri-Golgi vesicle found to contain DAB-precipitate. (MOV 2479 kb)
Supplementary Video 2
A detailed 3D representation of the peri-Golgi vesicle found to contain DAB-precipitate and adjacent cisternae. Note the small protrusions that extend away from the cisternae towards the vesicle. (MOV 1391 kb)
Rights and permissions
About this article
Cite this article
Grabenbauer, M., Geerts, W., Fernadez-Rodriguez, J. et al. Correlative microscopy and electron tomography of GFP through photooxidation. Nat Methods 2, 857–862 (2005). https://doi.org/10.1038/nmeth806
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth806