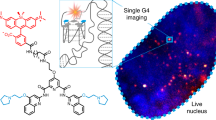
Abstract
DNA methyltransferases have a central role in the complex regulatory network of epigenetic modifications controlling gene expression in mammalian cells. To study the regulation of DNA methylation in living cells, we developed a trapping assay using transiently expressed fluorescent DNA methyltransferase 1 (Dnmt1) fusions and mechanism-based inhibitors 5-azacytidine (5-aza-C) or 5-aza-2′-deoxycytidine (5-aza-dC). These nucleotide analogs are incorporated into the newly synthesized DNA at nuclear replication sites and cause irreversible immobilization, that is, trapping of Dnmt1 fusions at these sites. We measured trapping by either fluorescence bleaching assays or photoactivation of photoactivatable green fluorescent protein fused to Dnmt1 (paGFP-Dnmt1) in mouse and human cells; mutations affecting the catalytic center of Dnmt1 prevented trapping. This trapping assay monitors kinetic properties and activity-dependent immobilization of DNA methyltransferases in their native environment, and makes it possible to directly compare mutations and inhibitors that affect regulation and catalytic activity of DNA methyltransferases in single living cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Li, E. Chromatin modification and epigenetic reprogramming in mammalian development. Nat. Rev. Genet. 3, 662–673 (2002).
Jaenisch, R. & Bird, A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. 33 (Suppl.), 245–254 (2003).
Bestor, T.H. The DNA methyltransferases of mammals. Hum. Mol. Genet. 9, 2395–2402 (2000).
Robertson, K.D. DNA methylation and chromatin—unraveling the tangled web. Oncogene 21, 5361–5379 (2002).
Tang, L.Y. et al. The eukaryotic DNMT2 genes encode a new class of cytosine-5 DNA methyltransferases. J. Biol. Chem. 278, 33613–33616 (2003).
Hermann, A., Schmitt, S. & Jeltsch, A. The human Dnmt2 has residual DNA-(cytosine-C5) methyltransferase activity. J. Biol. Chem. 278, 31717–31721 (2003).
Kunert, N., Marhold, J., Stanke, J., Stach, D. & Lyko, F.A. Dnmt2-like protein mediates DNA methylation in Drosophila. Development 130, 5083–5090 (2003).
Leonhardt, H., Page, A.W., Weier, H.U. & Bestor, T.H. A targeting sequence directs DNA methyltransferase to sites of DNA replication in mammalian nuclei. Cell 71, 865–873 (1992).
Chuang, L.S. et al. Human DNA-(cytosine-5) methyltransferase-PCNA complex as a target for p21WAF1. Science 277, 1996–2000 (1997).
Rountree, M.R., Bachman, K.E. & Baylin, S.B. DNMT1 binds HDAC2 and a new co-repressor, DMAP1, to form a complex at replication foci. Nat. Genet. 25, 269–277 (2000).
Robertson, K.D. et al. DNMT1 forms a complex with Rb, E2F1 and HDAC1 and represses transcription from E2F-responsive promoters. Nat. Genet. 25, 338–342 (2000).
Fuks, F., Hurd, P.J., Deplus, R. & Kouzarides, T. The DNA methyltransferases associate with HP1 and the SUV39H1 histone methyltransferase. Nucleic Acids Res. 31, 2305–2312 (2003).
Kimura, H. & Shiota, K. Methyl-CpG-binding protein, MeCP2, is a target molecule for maintenance DNA methyltransferase, Dnmt1. J. Biol. Chem. 278, 4806–4812 (2003).
Esteve, P.O., Chin, H.G. & Pradhan, S. Human maintenance DNA (cytosine-5)-methyltransferase and p53 modulate expression of p53-repressed promoters. Proc. Natl. Acad. Sci. USA 102, 1000–1005 (2005).
Jones, P.A. & Baylin, S.B. The fundamental role of epigenetic events in cancer. Nat. Rev. Genet. 3, 415–428 (2002).
Gaudet, F. et al. Induction of tumors in mice by genomic hypomethylation. Science 300, 489–492 (2003).
Rhee, I. et al. CpG methylation is maintained in human cancer cells lacking DNMT1. Nature 404, 1003–1007 (2000).
Cheng, X. & Roberts, R.J. AdoMet-dependent methylation, DNA methyltransferases and base flipping. Nucleic Acids Res. 29, 3784–3795 (2001).
Bestor, T.H. & Verdine, G.L. DNA methyltransferases. Curr. Opin. Cell Biol. 6, 380–389 (1994).
Santi, D.V., Garrett, C.E. & Barr, P.J. On the mechanism of inhibition of DNA-cytosine methyltransferases by cytosine analogs. Cell 33, 9–10 (1983).
Chen, L. et al. Direct identification of the active-site nucleophile in a DNA (cytosine-5)-methyltransferase. Biochemistry 30, 11018–11025 (1991).
Christman, J.K. 5-Azacytidine and 5-aza-2′-deoxycytidine as inhibitors of DNA methylation: mechanistic studies and their implications for cancer therapy. Oncogene 21, 5483–5495 (2002).
Easwaran, H.P., Schermelleh, L., Leonhardt, H. & Cardoso, M.C. Replication-independent chromatin loading of Dnmt1 during G2 and M phases. EMBO Rep. 5, 1181–1186 (2004).
Leonhardt, H. et al. Dynamics of DNA replication factories in living cells. J. Cell Biol. 149, 271–280 (2000).
Sporbert, A., Gahl, A., Ankerhold, R., Leonhardt, H. & Cardoso, M.C. DNA polymerase clamp shows little turnover at established replication sites but sequential de novo assembly at adjacent origin clusters. Mol. Cell 10, 1355–1365 (2002).
Wyszynski, M.W., Gabbara, S. & Bhagwat, A.S. Substitutions of a cysteine conserved among DNA cytosine methylases result in a variety of phenotypes. Nucleic Acids Res. 20, 319–326 (1992).
Jones, P.A. & Taylor, S.M. Cellular differentiation, cytidine analogs and DNA methylation. Cell 20, 85–93 (1980).
Cihak, A. Biological effects of 5-azacytidine in eukaryotes. Oncology 30, 405–422 (1974).
Patterson, G.H. & Lippincott-Schwartz, J. A photoactivatable GFP for selective photolabeling of proteins and cells. Science 297, 1873–1877 (2002).
Robert, M.F. et al. DNMT1 is required to maintain CpG methylation and aberrant gene silencing in human cancer cells. Nat. Genet. 33, 61–65 (2003).
Weisenberger, D.J. et al. Role of the DNA methyltransferase variant DNMT3b3 in DNA methylation. Mol. Cancer Res. 2, 62–72 (2004).
Liu, K., Wang, Y.F., Cantemir, C. & Muller, M.T. Endogenous assays of DNA methyltransferases: Evidence for differential activities of DNMT1, DNMT2, and DNMT3 in mammalian cells in vivo. Mol. Cell. Biol. 23, 2709–2719 (2003).
Juttermann, R., Li, E. & Jaenisch, R. Toxicity of 5-aza-2′-deoxycytidine to mammalian cells is mediated primarily by covalent trapping of DNA methyltransferase rather than DNA demethylation. Proc. Natl. Acad. Sci. USA 91, 11797–11801 (1994).
Easwaran, H.P., Leonhardt, H. & Cardoso, M.C. Cell cycle markers for live cell analyses. Cell Cycle 4, 453–455 (2005).
Cardoso, M.C. et al. Mapping and use of a sequence that targets DNA ligase I to sites of DNA replication in vivo. J. Cell Biol. 139, 579–587 (1997).
Sporbert, A., Domaing, P., Leonhardt, H. & Cardoso, M.C. PCNA acts as a stationary loading platform for transiently interacting Okazaki fragment maturation proteins. Nucleic Acids Res. 33, 3521–3528 (2005).
Campbell, R.E. et al. A monomeric red fluorescent protein. Proc. Natl. Acad. Sci. USA 99, 7877–7882 (2002).
Acknowledgements
We thank R.Y. Tsien for providing mRFP1 cDNA, J. Lippincott-Schwartz for providing paGFP cDNA, E. Li for mutant Dnmt1 ES cells and P. Vertino for the human DNMT1 cDNA. We thank M. Grohmann for sharing expression constructs. We are grateful to I. Grunewald and A. Gahl for technical assistance. This work was supported by grants from the Deutsche Forschungsgemeinschaft and the Max Delbrück Center to H.L. and M.C.C.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
GFP-Dnmt1 fusion protein restores methylation at chromocenters in dnmt1−/− ES cells. (PDF 399 kb)
Supplementary Fig. 2
Trapping of DNMT1 after 5–aza–dC treatment in human SH–EP N14 neuroblastoma cells. (PDF 204 kb)
Supplementary Video 1
Dnmt1 mobility before and during 5–aza–dC treatment in a single living cell. (MOV 2476 kb)
Rights and permissions
About this article
Cite this article
Schermelleh, L., Spada, F., Easwaran, H. et al. Trapped in action: direct visualization of DNA methyltransferase activity in living cells. Nat Methods 2, 751–756 (2005). https://doi.org/10.1038/nmeth794
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth794
This article is cited by
-
DNA damage, demethylation and anticancer activity of DNA methyltransferase (DNMT) inhibitors
Scientific Reports (2023)
-
The DNMT1 inhibitor GSK-3484862 mediates global demethylation in murine embryonic stem cells
Epigenetics & Chromatin (2021)
-
Quantitative 3D structured illumination microscopy of nuclear structures
Nature Protocols (2017)
-
DNA methylation requires a DNMT1 ubiquitin interacting motif (UIM) and histone ubiquitination
Cell Research (2015)
-
Identification of direct targets and modified bases of RNA cytosine methyltransferases
Nature Biotechnology (2013)