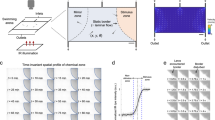
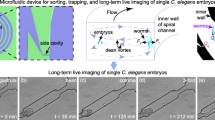
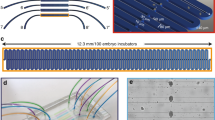
Abstract
The nematode C. elegans is an excellent model organism for studying behavior at the neuronal level. Because of the organism's small size, it is challenging to deliver stimuli to C. elegans and monitor neuronal activity in a controlled environment. To address this problem, we developed two microfluidic chips, the 'behavior' chip and the 'olfactory' chip for imaging of neuronal and behavioral responses in C. elegans. We used the behavior chip to correlate the activity of AVA command interneurons with the worm locomotion pattern. We used the olfactory chip to record responses from ASH sensory neurons exposed to high-osmotic-strength stimulus. Observation of neuronal responses in these devices revealed previously unknown properties of AVA and ASH neurons. The use of these chips can be extended to correlate the activity of sensory neurons, interneurons and motor neurons with the worm's behavior.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Lockery, S.R. & Goodman, M.B. Tight-seal whole-cell patch clamping of Caenorhabditis elegans neurons. Methods Enzymol. 293, 201–217 (1998).
Pologruto, T.A., Yasuda, R. & Svoboda, K. Monitoring neural activity and [Ca2+] with genetically encoded Ca2+ indicators. J. Neurosci. 24, 9572–9579 (2004).
Kerr, R. et al. Optical imaging of calcium transients in neurons and pharyngeal muscle of C. elegans. Neuron 26, 583–594 (2000).
Suzuki, H. et al. In vivo imaging of C. elegans mechanosensory neurons demonstrates a specific role for the MEC-4 channel in the process of gentle touch sensation. Neuron 39, 1005–1017 (2003).
Hilliard, M.A. et al. In vivo imaging of C. elegans ASH neurons: cellular response and adaptation to chemical repellents. EMBO J. 24, 63–72 (2005).
Faumont, S. & Lockery, S.R. The awake behaving worm: simultaneous imaging of neuronal activity and behavior in intact animals at millimeter scale. J. Neurophysiol. 95, 1976–1981 (2006).
Clark, D.A., Gabel, C.V., Gabel, H. & Samuel, A.D.T. Temporal activity patterns in thermosensory neurons of freely moving C. elegans encode spatial thermal gradients. J. Neurosci. 27, 6083–6090 (2007).
Thorsen, T., Maerkl, S.J. & Quake, S.R. Microfluidic large-scale integration. Science 298, 580–584 (2002).
Griffith, L.G. & Naughton, G. Tissue engineering—current challenges and expanding opportunities. Science 295, 1009–1014 (2002).
Wheeler, A.R. et al. Microfluidic device for single-cell analysis. Anal. Chem. 75, 3581–3586 (2003).
Lucchetta, E.M., Lee, J.H., Fu, L.A., Patel, N.H. & Ismagilov, R.F. Dynamics of Drosophila embryonic patterning network perturbed in space and time using microfluidics. Nature 434, 1134–1138 (2005).
Younan, X. & Whitesides, G. Soft lithography. Annu. Rev. Materials Sci. 28, 153–184 (1998).
Croll, A. Components and patterns in the behavior of the nematode Caenorhabditis elegans. J. Zool. 176, 159–176 (1975).
Gray, J. & Lissmann, H.W. The locomotion of nematodes. J. Exp. Biol. 41, 135–154 (1964).
Chalfie, M. et al. The neural circuit for touch sensitivity in Caenorhabditis elegans. J. Neurosci. 5, 956–964 (1985).
Nakai, J., Ohkura, M. & Imoto, K. A high signal-to-noise Ca2+ probe composed of a single green fluorescent protein. Nat. Biotechnol. 19, 137–141 (2001).
Chesler, M. & Kaila, K. Modulation of pH by neuronal activity. Trends Neurosci. 15, 396–402 (1992).
Kneen, M., Farinas, J., Li, Y. & Verkman, A.S. Green fluorescent protein as a noninvasive intracellular pH indicator. Biophys. J. 74, 1591–1599 (1998).
Brenner, S. The genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974).
Kaplan, J.M. & Horvitz, H.R. A dual mechanosensory and chemosensory neuron in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 90, 2227–2231 (1993).
Nagai, T. et al. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 20, 87–90 (2002).
Jager, E.W., Smela, E. & Inganas, O. Microfabricating conjugated polymer actuators. Science 290, 1540–1545 (2000).
Gaitan, M. & Locascio, L.E. Embedded microheating elements in polymeric micro channels for temperature control and fluid flow sensing. J. Res. Natl. Inst. Stand. Technol. 109, 335–344 (2004).
Kahn-Kirby, A.H. et al. Specific polyunsaturated fatty acids drive TRPV-dependent sensory signaling in vivo. Cell 119, 889–900 (2004).
Mello, C.C., Kramer, J.M., Stinchcomb, D. & Ambros, V. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10, 3959–3970 (1991).
Brockie, P.J., Madsen, D.M., Zheng, Y., Mellem, J. & Maricq, A.V. Differential expression of glutamate receptor subunits in the nervous system of Caenorhabditis elegans and their regulation by the homeodomain protein UNC-42. J. Neurosci. 21, 1510–1522 (2001).
Acknowledgements
We thank S. Leibler for the use of his clean room facility. This work was supported by the Howard Hughes Medical Institute and a fellowship from the International Human Frontier Science Program Organization to M.Z. C.I.B. is a Howard Hughes Medical Institute investigator.
Author information
Authors and Affiliations
Contributions
N.C. designed the microfluidic chips, conducted the experiments, interpreted the data and wrote the paper; M.Z. designed and conducted the experiments, interpreted the data and wrote the paper; C.I.B. interpreted the data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–2 (PDF 879 kb)
Supplementary Video 1
AVA calcium transients correlate with the generation of anterior-traveling body waves. (MOV 2355 kb)
Rights and permissions
About this article
Cite this article
Chronis, N., Zimmer, M. & Bargmann, C. Microfluidics for in vivo imaging of neuronal and behavioral activity in Caenorhabditis elegans. Nat Methods 4, 727–731 (2007). https://doi.org/10.1038/nmeth1075
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth1075
This article is cited by
-
Molecular and circuit mechanisms underlying avoidance of rapid cooling stimuli in C. elegans
Nature Communications (2024)
-
Pathogenic bacteria modulate pheromone response to promote mating
Nature (2023)
-
Dissecting the genetic landscape of GPCR signaling through phenotypic profiling in C. elegans
Nature Communications (2023)
-
An optofluidic platform for interrogating chemosensory behavior and brainwide neural representation in larval zebrafish
Nature Communications (2023)
-
Molecular encoding and synaptic decoding of context during salt chemotaxis in C. elegans
Nature Communications (2022)