Abstract
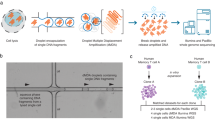
Single-cell genomics is critical for understanding cellular heterogeneity in cancer, but existing library preparation methods are expensive, require sample preamplification and introduce coverage bias. Here we describe direct library preparation (DLP), a robust, scalable, and high-fidelity method that uses nanoliter-volume transposition reactions for single-cell whole-genome library preparation without preamplification. We examined 782 cells from cell lines and triple-negative breast xenograft tumors. Low-depth sequencing, compared with existing methods, revealed greater coverage uniformity and more reliable detection of copy-number alterations. Using phylogenetic analysis, we found minor xenograft subpopulations that were undetectable by bulk sequencing, as well as dynamic clonal expansion and diversification between passages. Merging single-cell genomes in silico, we generated 'bulk-equivalent' genomes with high depth and uniform coverage. Thus, low-depth sequencing of DLP libraries may provide an attractive replacement for conventional bulk sequencing methods, permitting analysis of copy number at the cell level and of other genomic variants at the population level.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Nowell, P.C. The clonal evolution of tumor cell populations. Science 194, 23–28 (1976).
Aparicio, S. & Caldas, C. The implications of clonal genome evolution for cancer medicine. N. Engl. J. Med. 368, 842–851 (2013).
Burrell, R.A., McGranahan, N., Bartek, J. & Swanton, C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature 501, 338–345 (2013).
Shah, S.P. et al. Mutational evolution in a lobular breast tumour profiled at single nucleotide resolution. Nature 461, 809–813 (2009).
Yachida, S. et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 467, 1114–1117 (2010).
Campbell, P.J. et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature 467, 1109–1113 (2010).
Gerlinger, M. et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 366, 883–892 (2012).
Ding, L. et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature 481, 506–510 (2012).
Landau, D.A. et al. Evolution and impact of subclonal mutations in chronic lymphocytic leukemia. Cell 152, 714–726 (2013).
Gundem, G. et al. The evolutionary history of lethal metastatic prostate cancer. Nature 520, 353–357 (2015).
Eirew, P. et al. Dynamics of genomic clones in breast cancer patient xenografts at single-cell resolution. Nature 518, 422–426 (2015).
Navin, N. et al. Tumour evolution inferred by single-cell sequencing. Nature 472, 90–94 (2011).
Zong, C., Lu, S., Chapman, A.R. & Xie, X.S. Genome-wide detection of single-nucleotide and copy-number variations of a single human cell. Science 338, 1622–1626 (2012).
Hou, Y. et al. Single-cell exome sequencing and monoclonal evolution of a JAK2-negative myeloproliferative neoplasm. Cell 148, 873–885 (2012).
Ni, X. et al. Reproducible copy number variation patterns among single circulating tumor cells of lung cancer patients. Proc. Natl. Acad. Sci. USA 110, 21083–21088 (2013).
Gawad, C., Koh, W. & Quake, S.R. Dissecting the clonal origins of childhood acute lymphoblastic leukemia by single-cell genomics. Proc. Natl. Acad. Sci. USA 111, 17947–17952 (2014).
Lohr, J.G. et al. Whole-exome sequencing of circulating tumor cells provides a window into metastatic prostate cancer. Nat. Biotechnol. 32, 479–484 (2014).
Wang, Y. et al. Clonal evolution in breast cancer revealed by single nucleus genome sequencing. Nature 512, 155–160 (2014).
Baslan, T. et al. Optimizing sparse sequencing of single cells for highly multiplex copy number profiling. Genome Res. 25, 714–724 (2015).
Gao, R. et al. Punctuated copy number evolution and clonal stasis in triple-negative breast cancer. Nat. Genet. 48, 1119–1130 (2016).
Roth, A. et al. PyClone: statistical inference of clonal population structure in cancer. Nat. Methods 11, 396–398 (2014).
Gerstung, M. et al. Reliable detection of subclonal single-nucleotide variants in tumour cell populations. Nat. Commun. 3, 811 (2012).
Ha, G. et al. TITAN: inference of copy number architectures in clonal cell populations from tumor whole-genome sequence data. Genome Res. 24, 1881–1893 (2014).
Falconer, E. et al. DNA template strand sequencing of single-cells maps genomic rearrangements at high resolution. Nat. Methods 9, 1107–1112 (2012).
Wang, J., Fan, H.C., Behr, B. & Quake, S.R. Genome-wide single-cell analysis of recombination activity and de novo mutation rates in human sperm. Cell 150, 402–412 (2012).
de Bourcy, C.F. et al. A quantitative comparison of single-cell whole genome amplification methods. PLoS One 9, e105585 (2014).
Macaulay, I.C. & Voet, T. Single cell genomics: advances and future perspectives. PLoS Genet. 10, e1004126 (2014).
Garvin, T. et al. Interactive analysis and assessment of single-cell copy-number variations. Nat. Methods 12, 1058–1060 (2015).
Leung, M.L. et al. Highly multiplexed targeted DNA sequencing from single nuclei. Nat. Protoc. 11, 214–235 (2016).
van den Bos, H. et al. Single-cell whole genome sequencing reveals no evidence for common aneuploidy in normal and Alzheimers disease neurons. Genome Biol. 17, 116 (2016).
Adey, A. et al. Rapid, low-input, low-bias construction of shotgun fragment libraries by high-density in vitro transposition. Genome Biol. 11, R119 (2010).
Burleigh, A. et al. A co-culture genome-wide RNAi screen with mammary epithelial cells reveals transmembrane signals required for growth and differentiation. Breast Cancer Res. 17, 4 (2015).
International HapMap Consortium. A haplotype map of the human genome. Nature 437, 1299–1320 (2005).
Ha, G. et al. Integrative analysis of genome-wide loss of heterozygosity and monoallelic expression at nucleotide resolution reveals disrupted pathways in triple-negative breast cancer. Genome Res. 22, 1995–2007 (2012).
Baslan, T. et al. Genome-wide copy number analysis of single cells. Nat. Protoc. 7, 1024–1041 (2012).
Ronquist, F. & Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574 (2003).
Knouse, K.A., Wu, J. & Amon, A. Assessment of megabase-scale somatic copy number variation using single-cell sequencing. Genome Res. 26, 376–384 (2016).
Ding, J. et al. Feature-based classifiers for somatic mutation detection in tumour-normal paired sequencing data. Bioinformatics 28, 167–175 (2012).
McPherson, A. et al. nFuse: discovery of complex genomic rearrangements in cancer using high-throughput sequencing. Genome Res. 22, 2250–2261 (2012).
McConnell, M.J. et al. Mosaic copy number variation in human neurons. Science 342, 632–637 (2013).
Cai, X. et al. Single-cell, genome-wide sequencing identifies clonal somatic copy-number variation in the human brain. Cell Rep. 8, 1280–1289 (2014).
Knouse, K.A., Wu, J., Whittaker, C.A. & Amon, A. Single cell sequencing reveals low levels of aneuploidy across mammalian tissues. Proc. Natl. Acad. Sci. USA 111, 13409–13414 (2014).
Mazutis, L. et al. Multi-step microfluidic droplet processing: kinetic analysis of an in vitro translated enzyme. Lab Chip 9, 2902–2908 (2009).
Mazutis, L. et al. Single-cell analysis and sorting using droplet-based microfluidics. Nat. Protoc. 8, 870–891 (2013).
Macosko, E.Z. et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202–1214 (2015).
Rotem, A. et al. Single-cell ChIP-seq reveals cell subpopulations defined by chromatin state. Nat. Biotechnol. 33, 1165–1172 (2015).
Huft, J., Da Costa, D.J., Walker, D. & Hansen, C.L. Three-dimensional large-scale microfluidic integration by laser ablation of interlayer connections. Lab Chip 10, 2358–2365 (2010).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
Acknowledgements
We gratefully acknowledge funding support from the BC Cancer Foundation, the Canadian Breast Cancer Foundation, Genome Canada/Genome BC, the Natural Sciences & Engineering Research Council of Canada (grant RGPIN 386152-10 to C.L.H.), the Terry Fox Research Institute (grant NFP 1021 to S.A. and S.P.S.), the Canadian Institutes of Health Research (grant MOP 126119 to S.A. and S.P.S.), and the Canadian Cancer Society Research Institute (grant 701584 to S.A. and S.P.S.). S.A. and S.P.S. are supported as Canada Research Chairs, and S.P.S. is supported as a Michael Smith Foundation for Health Research Scholar. H.Z. and A.S. are each supported by a Vanier Canada Graduate Scholarship.
Author information
Authors and Affiliations
Contributions
H.Z., A.S., S.P.S., S.A., and C.L.H. designed the research. H.Z. performed experiments. A.S. analyzed the data. A.S., H.Z., C.L.H., S.A., and S.P.S. wrote the paper. E.L. prepared tissue samples and bulk libraries. P.E. performed xenograft transplants. M.V. contributed to technology development. C.L.H., S.A., and S.P.S. supervised the research.
Corresponding authors
Ethics declarations
Competing interests
C.L.H., H.Z., A.S., S.A. and S.P.S. are inventors on a patent application covering elements of the technology described here and have a financial interest through revenue-sharing policies of the University of British Columbia (UBC). C.L.H. has a financial interest in AbCellera, a company that has licensed rights from UBC to the aforementioned patent application.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–14, Supplementary Tables 1 and 6, and Supplementary Note (PDF 15838 kb)
Supplementary Table 2
DLP single-cell sequencing metrics for immortalized normal cell lines 184-hTERT-L2 (page 1) and GM18507 (page 2). (XLS 87 kb)
Supplementary Table 3
DLP single-cell sequencing metrics for patient-derived triple-negative breast cancer xenograft tumours SA501X3F (page 1) and SA501X4F (page 2). (XLS 170 kb)
Supplementary Table 4
Statistics table with Kruskal–Wallis tests (page 1) and Pearson's correlations (page 2). (XLS 22 kb)
Supplementary Table 5
Sequencing metrics for DLP merged bulk-equivalent and standard bulk genomes. (XLS 16 kb)
Supplementary Data
Microfluidic device AutoCAD design file. (ZIP 786 kb)
Rights and permissions
About this article
Cite this article
Zahn, H., Steif, A., Laks, E. et al. Scalable whole-genome single-cell library preparation without preamplification. Nat Methods 14, 167–173 (2017). https://doi.org/10.1038/nmeth.4140
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.4140