Abstract
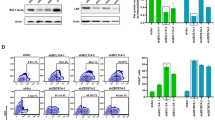
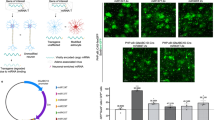
We present a flexible and highly specific targeting method for lentiviral vectors based on single-chain antibodies recognizing cell-surface antigens. We generated lentiviral vectors specific for human CD105+ endothelial cells, human CD133+ hematopoietic progenitors and mouse GluA-expressing neurons. Lentiviral vectors specific for CD105 or for CD20 transduced their target cells as efficiently as VSV-G pseudotyped vectors but discriminated between endothelial cells and lymphocytes in mixed cultures. CD133-targeted vectors transduced CD133+ cultured hematopoietic progenitor cells more efficiently than VSV-G pseudotyped vectors, resulting in stable long-term transduction. Lentiviral vectors targeted to the glutamate receptor subunits GluA2 and GluA4 exhibited more than 94% specificity for neurons in cerebellar cultures and when injected into the adult mouse brain. We observed neuron-specific gene modification upon transfer of the Cre recombinase gene into the hippocampus of reporter mice. This approach allowed targeted gene transfer to many cell types of interest with an unprecedented degree of specificity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Cockrell, A.S. & Kafri, T. Gene delivery by lentivirus vectors. Mol. Biotechnol. 36, 184–204 (2007).
Singer, O. & Verma, I.M. Applications of lentiviral vectors for shRNA delivery and transgenesis. Curr. Gene Ther. 8, 483–488 (2008).
Naldini, L. Medicine. A comeback for gene therapy. Science 326, 805–806 (2009).
Frecha, C., Szecsi, J., Cosset, F.L. & Verhoeyen, E. Strategies for targeting lentiviral vectors. Curr. Gene Ther. 8, 449–460 (2008).
Wong, L.F. et al. Lentivirus-mediated gene transfer to the central nervous system: therapeutic and research applications. Hum. Gene Ther. 17, 1–9 (2006).
Meunier, A. & Pohl, M. Lentiviral vectors for gene transfer into the spinal cord glial cells. Gene Ther. 16, 476–482 (2009).
Buchholz, C.J., Muhlebach, M.D. & Cichutek, K. Lentiviral vectors with measles virus glycoproteins—dream team for gene transfer? Trends Biotechnol. 27, 259–265 (2009).
Morizono, K. et al. Lentiviral vector retargeting to P-glycoprotein on metastatic melanoma through intravenous injection. Nat. Med. 11, 346–352 (2005).
Yang, L., Bailey, L., Baltimore, D. & Wang, P. Targeting lentiviral vectors to specific cell types in vivo. Proc. Natl. Acad. Sci. USA 103, 11479–11484 (2006).
Nakamura, T. et al. Rescue and propagation of fully retargeted oncolytic measles viruses. Nat. Biotechnol. 23, 209–214 (2005).
Funke, S. et al. Targeted cell entry of lentiviral vectors. Mol. Ther. 16, 1427–1436 (2008).
Funke, S. et al. Pseudotyping lentiviral vectors with the wild-type measles virus glycoproteins improves titer and selectivity. Gene Ther. 16, 700–705 (2009).
Fonsatti, E. & Maio, M. Highlights on endoglin (CD105): from basic findings towards clinical applications in human cancer. J. Transl. Med. 2, 18 (2004).
Shmelkov, S.V., St Clair, R., Lyden, D. & Rafii, S. AC133/CD133/Prominin-1. Int. J. Biochem. Cell Biol. 37, 715–719 (2005).
Mizrak, D., Brittan, M. & Alison, M.R. CD133: molecule of the moment. J. Pathol. 214, 3–9 (2008).
Yanagi, Y., Takeda, M., Ohno, S. & Hashiguchi, T. Measles virus receptors. Curr. Top. Microbiol. Immunol. 329, 13–30 (2009).
Baum, C., Hegewisch-Becker, S., Eckert, H.G., Stocking, C. & Ostertag, W. Novel retroviral vectors for efficient expression of the multidrug resistance (mdr-1) gene in early hematopoietic cells. J. Virol. 69, 7541–7547 (1995).
Pasternack, A. et al. Alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor channels lacking the N-terminal domain. J. Biol. Chem. 277, 49662–49667 (2002).
Carman, C.V. & Springer, T.A. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J. Cell Biol. 167, 377–388 (2004).
Hess, K.L. et al. Human and murine high endothelial venule cells phagocytose apoptotic leukocytes. Exp. Cell Res. 236, 404–411 (1997).
Ozawa, S., Kamiya, H. & Tsuzuki, K. Glutamate receptors in the mammalian central nervous system. Prog. Neurobiol. 54, 581–618 (1998).
Sprengel, R. Role of AMPA receptors in synaptic plasticity. Cell Tissue Res. 326, 447–455 (2006).
Fuchs, E.C. et al. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron 53, 591–604 (2007).
Soriano, P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70–71 (1999).
Rolls, M.M., Webster, P., Balba, N.H. & Rose, J.K. Novel infectious particles generated by expression of the vesicular stomatitis virus glycoprotein from a self-replicating RNA. Cell 79, 497–506 (1994).
Kim, Y.S. et al. Transduction of human primitive repopulating hematopoietic cells with lentiviral vectors pseudotyped with various envelope proteins. Mol. Ther. 18, 1310–1317 (2010).
Pariente, N., Mao, S.H., Morizono, K. & Chen, I.S. Efficient targeted transduction of primary human endothelial cells with dual-targeted lentiviral vectors. J. Gene Med. 10, 242–248 (2008).
Lang, P. et al. Transplantation of a combination of CD133+ and CD34+ selected progenitor cells from alternative donors. Br. J. Haematol. 124, 72–79 (2004).
Singh, S.K. et al. Identification of human brain tumour initiating cells. Nature 423, 396–401 (2004).
Brown, B.D. & Naldini, L. Exploiting and antagonizing microRNA regulation for therapeutic and experimental applications. Nat. Rev. Genet. 10, 578–585 (2009).
Jespersen, L.K., Kuusinen, A., Orellana, A., Keinanen, K. & Engberg, J. Use of proteoliposomes to generate phage antibodies against native AMPA receptor. Eur. J. Biochem. 267, 1382–1389 (2000).
Volkel, T., Muller, R. & Kontermann, R.E. Isolation of endothelial cell-specific human antibodies from a novel fully synthetic scFv library. Biochem. Biophys. Res. Commun. 317, 515–521 (2004).
Demaison, C. et al. High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency (correction of immunodeficiency) virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum. Gene Ther. 13, 803–813 (2002).
Sun, Y., Finger, C., Alvarez-Vallina, L., Cichutek, K. & Buchholz, C.J. Chronic gene delivery of interferon-inducible protein 10 through replication-competent retrovirus vectors suppresses tumor growth. Cancer Gene Ther. 12, 900–912 (2005).
Steinbach, J.P. et al. Hypersensitivity to seizures in beta-amyloid precursor protein deficient mice. Cell Death Differ. 5, 858–866 (1998).
Liehl, B. et al. Simian immunodeficiency virus vector pseudotypes differ in transduction efficiency and target cell specificity in brain. Gene Ther. 14, 1330–1343 (2007).
Fuchs, E.C. et al. Genetically altered AMPA-type glutamate receptor kinetics in interneurons disrupt long-range synchrony of gamma oscillation. Proc. Natl. Acad. Sci. USA 98, 3571–3576 (2001).
Cetin, A., Komai, S., Eliava, M., Seeburg, P.H. & Osten, P. Stereotaxic gene delivery in the rodent brain. Nat. Protoc. 1, 3166–3173 (2006).
Acknowledgements
This work was supported by grants from the Priority Programme “Mechanisms of gene vector entry and persistence” of the Deutsche Forschungsgemeinschaft to C.J.B. and K.C. and from the 7th European Community programme project Persisting Transgenesis (Persist) to C.J.B. U.C.M. was supported by grants of the Deutsche Forschungsgemeinschaft (MU 1457/8-1) and the “Nationales Genomforschungsnetz” (01GS08128). T.A. is supported by the graduate study program GK1172 Biologicals of the Goethe University Frankfurt AM.
Author information
Authors and Affiliations
Contributions
B.A., T.A. and S.K. designed and performed experiments and contributed to writing of the manuscript. J.H., A.C., J.B., I.C.S., R.C.M. and H.P. performed experiments. R.E.K., U.K., I.C.D.J. and K.K. contributed protocols and reagents. C.H. and H.M. supervised work. U.C.M. supervised work and contributed to writing of the manuscript. K.C. acquired grants. C.J.B. conceived and designed the study, acquired grants, supervised work and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
I.C.D.J. is an employee of Miltenyi Biotec GmbH. S.K., K.C. and C.J.B. are listed as inventors in an international Patent Cooperation Treaty European patent application (PCT/EP2007/008384) assigned to the Paul Ehrlich Institut, which includes as claims the generation of targeted lentiviral vectors.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12, Supplementary Tables 1–2 (PDF 928 kb)
Rights and permissions
About this article
Cite this article
Anliker, B., Abel, T., Kneissl, S. et al. Specific gene transfer to neurons, endothelial cells and hematopoietic progenitors with lentiviral vectors. Nat Methods 7, 929–935 (2010). https://doi.org/10.1038/nmeth.1514
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.1514
This article is cited by
-
High-Voltage-Activated Calcium Channel in the Afferent Pain Pathway: An Important Target of Pain Therapies
Neuroscience Bulletin (2019)
-
Antigen-presenting cell-targeted lentiviral vectors do not support the development of productive T-cell effector responses: implications for in vivo targeted vaccine delivery
Gene Therapy (2017)
-
Method for Dual Viral Vector Mediated CRISPR-Cas9 Gene Disruption in Primary Human Endothelial Cells
Scientific Reports (2017)
-
Highly efficient baculovirus-mediated multigene delivery in primary cells
Nature Communications (2016)
-
A scalable method to concentrate lentiviral vectors pseudotyped with measles virus glycoproteins
Gene Therapy (2015)