Abstract
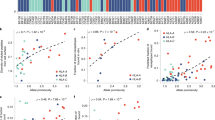
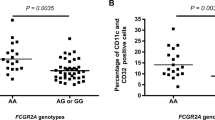
The highly polymorphic human leukocyte antigen (HLA) class I molecules help to determine the specificity and repertoire of the immune response. The great diversity of these antigen-binding molecules confers differential advantages in responding to pathogens, but presents a major obstacle to distinguishing HLA allele–specific effects. HLA class I supertypes provide a functional classification for the many different HLA alleles that overlap in their peptide-binding specificities. We analyzed the association of these discrete HLA supertypes with HIV disease progression rates in a population of HIV-infected men. We found that HLA supertypes alone and in combination conferred a strong differential advantage in responding to HIV infection, independent of the contribution of single HLA alleles that associate with progression of the disease. The correlation of the frequency of the HLA supertypes with viral load suggests that HIV adapts to the most frequent alleles in the population, providing a selective advantage for those individuals who express rare alleles.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Trowsdale, J. & Campbell, R.D. Complexity in the major histocompatibility complex. Eur. J. Immunogenet. 19, 45–55 (1992).
Bjorkman, P.J. & Parham, P. Structure, function, and diversity of class I major histocompatibility complex molecules. Annu. Rev. Biochem. 59, 253–288 (1990).
Buus, S., Sette, A., Colon, S., Miles, C. & Grey, H.M. The relation between major histocompatibility complex (MHC) restriction and the capacity of Ia to bind immunogenetic peptides. Science 235, 1353–1358 (1987).
Hill, A.V.S. et al. Common West African HLA antigens are associated with protection from severe malaria. Nature 352, 595–600 (1991).
Little, A.M. & Parham, P. Polymorphism and evolution of HLA class I and II genes and molecules. Rev. Immunogenet. 1, 105–123 (1999).
Hughes, A.L., Ota, T. & Nei, M. Positive Darwinian selection promotes charge profile diversity in the antigen-binding cleft of class I major-histocompatibility-complex molecules. Mol. Biol. Evol. 7, 515–524 (1990).
Slatkin, M. & Muirhead, C.A. A method for estimating the intensity of overdominant selection from the distribution of allele frequencies. Genetics 156, 2119–2126 (2000).
Bodmer, W.F. Evolutionary significance of the HLA system. Nature 237, 139–145 (1972).
Hill, A.V.S. The immunogenetics of human infectious diseases. Ann. Rev. Immunol. 16, 593–617 (1998).
Propato, A. et al. Spreading of HIV-specific CD8+ T-cell repertoire in long-term nonprogressors and its role in the control of viral load and disease activity. Hum. Immunol. 62, 561–576 (2001).
MacDonald, K.S. et al. Human leucocyte antigen supertypes and immune susceptibility to HIV-1, implications for vaccine design. Immunol. Lett. 79, 151–157 (2001).
Bertoni, R. et al. Human histocompatibility leukocyte antigen-binding supermotifs predict broadly cross-reactive cytotoxic T lymphocyte responses in patients with acute hepatitis. J. Clin. Invest. 100, 503–513 (1997).
Tomiyama, H., Yamada, N., Komatsu, H., Hirayama, K. & Takiguchi, M. A single CTL clone can recognize a naturally processed HIV-1 epitope presented by two different HLA class I molecules. Eur. J. Immunol. 30, 2521–2530 (2000).
Altfeld, M.A. et al. Identification of novel HLA-A2-restricted human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte epitopes predicted by the HLA-A2 supertype peptide-binding motif. J. Virol. 75, 1301–1311 (2001).
Clarke, B. The ecological genetics of host-parasite relationships. in Genetic aspects of host-parasite relationships. (eds. Taylor, A.E.R. & Muller, R.M.) 87–103 (Blackwell Oxford, 1976).
Howard, J.C. MHC organization of the rat: evolutionary considerations. in Evolution and Vertebrate Immunity (eds. Kelsoe, G. & Schulze, D.H.) 397–411 (University of Texas Press,, Austin, 1987).
Roger, M. Influence of host genes on HIV-1 disease progression. FASEB J. 12, 625–632 (1998).
Trachtenberg, E.A. & Erlich, H.A. A review of the role of the human leukocyte antigen (HLA) system as a host immunogenetic factor influencing HIV transmission and course of infection with progression to AIDS. in HIV Molecular Immunology Database (eds. Korber, B. et al.) 1–60 (Theoretical Biology and Biophysics Group, Los Alamos National Laboratory, Los Alamos, NM., 2001).
Carrington, M. et al. HLA and HIV-1: heterozygote advantage and B*35-Cw*04 disadvantage. Science 283, 1748–1752 (1999).
Keet, I.P. et al. Consistent associations of HLA class I and II and transporter gene products with progression of human immunodeficiency virus type 1 infection in homosexual men. J. Infect. Dis. 180, 299–309 (1999).
Marsh, S.G.E., Parham, P. & Barber, L.D. The HLA FactsBook 398 (Academic Press, New York, 2002).
Sette, A. & Sidney, J. Nine major HLA class I supertypes account for the vast preponderance of HLA-A and -B polymorphism. Immunogenetics 50, 201–112 (1999).
Kaslow, R.A. et al. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat. Med. 2, 405–411 (1996).
Hendel, H. et al. New class I and II HLA alleles strongly associated with opposite patterns of progression to AIDS. J. Immunol. 162, 6942–6946 (1999).
Begovich, A.B. et al. Polymorphism, recombination and linkage disequilibrium within the HLA class II region. J. Immunol. 148, 249–258 (1992).
Goulder, P.J. et al. Evolution and transmission of stable CTL escape mutations in HIV infection. Nature 412, 334–338 (2001).
Rissanen, J. Stochastic Complexity in Statistical Inquiry. 171 (World Scientific (Singapore), 1989).
Rissanen, J. Hypothesis selection and testing by the MDL principle. Comput. J. 42, 260–269 (1999).
Li, M. & Vitanyi, P. An Introduction to Kolmogorov Complexity and its Applications. 546 (Springer-Verlag (New York), 1993).
Nelson, G.W., Kaslow, R. & Mann, D.L. Frequency of HLA allele-specific peptide motifs in HIV-1 proteins correlates with the allele's association with relative rates of disease progression after HIV-1 infection. Proc. Natl. Acad. Sci. USA 94, 9802–9807 (1997).
Anastos, K. et al. Association of race and gender with HIV-1 RNA levels and immunologic progression. J. Acquir. Immune Defic. Syndr. 24, 218–226 (2000).
Phair, J. et al. Acquired immune deficiency syndrome occurring within 5 years of infection with human immune deficiency virus type-1: the Multicenter AIDS Cohort Study. J. Acquir. Immune Defic. Syndr. 5, 490–496 (1992).
Saah, A.J. et al. Predictors of the risk of development of acquired immunodeficiency syndrome within 24 months among gay men seropositive for human immunodeficiency virus type 1: a report from the Multicenter AIDS Cohort Study. Am. J. Epidemiol. 135, 1147–1155 (1992).
Allen, T.M. et al. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature 407, 386–390 (2000).
van der Burg, S.H. et al. HIV-1 reverse transcriptase-specific CTL against conserved epitopes do not protect against progression to AIDS. J. Immunol. 159, 3648–3654 (1997).
Moore, C.B. et al. Evidence of HIV-1 adaptation to HLA-restricted immune responses at a population level. Science 296, 1439–1443 (2002).
Hill, A., Takiguchi, M. & McMichael, A. Different rates of HLA class I molecule assembly which are determined by amino acid sequence in the alpha 2 domain. Immunogenetics 37, 95–101 (1993).
Williams, A., Peh, C.A. & Elliott, T. The cell biology of MHC class I antigen presentation. Tissue Antigens 59, 3–17 (2002).
Boon, A.C. et al. The magnitude and specificity of influenza A virus-specific cytotoxic T-lymphocyte responses in humans is related to HLA-A and -B phenotype. J. Virol. 76, 582–590 (2002).
Yusim, K. et al. Clustering patterns of cytotoxic T-lymphocyte epitopes in human immunodeficiency virus type 1 (HIV-1) proteins reveal imprints of immune evasion on HIV-1 global variation. J. Virol. 76, 8757–8768 (2002).
de Groot, N.G. et al. Evidence for an ancient selective sweep in the MHC class I gene repertoire of chimpanzees. Proc. Natl. Acad. Sci. USA 99, 11748–11753 (2002).
Bugawan, T.L., Begovich, A.B. & Erlich, H.A. Rapid HLA-DPB typing using enzymatically amplified DNA and nonradioactive sequence specific oligonucleotide probes. Immunogenetics 34, 413 (1991).
Bugawan, T.L. & Erlich, H.A. Rapid typing of HLA-DQB1 DNA polymorphism using nonradioactive oligonucleotide probes and amplified DNA. Immunogenetics 33, 163–170 (1991).
Bugawan, T.L., Apple, R. & Erlich, H.A. A method for typing polymorphism at the HLA-A locus using PCR amplification and immobilized oligonucleotide probes. Tissue Antigens 44, 137–147 (1994).
Scharf, S.J., Griffith, R.L. & Erlich, H.A. Rapid typing of DNA sequence polymorphism at the HLA-DRB1 locus using the polymerase chain reaction and nonradioactive oligonucleotide probes. Hum. Immunol. 30, 190–201 (1991).
Erlich, H.A. & Trachtenberg E.A. PCR-based methods of HLA typing. in Molecular Epidemiology: A Practical Approach (eds. Carrington, M. & Hoelzel, A.R.) 181–207 (Oxford University Press, Oxford, 2001).
Cao, K. et al. Analysis of the frequencies of HLA-A, B, and C alleles and haplotypes in the five major ethnic groups of the United States reveals high levels of diversity in these loci and contrasting distribution patterns in these populations. Hum. Immunol. 62, 1009–1030 (2001).
Venables, W.N. & Ripley, B.D. Modern Applied Statistics with S-PLUS. 501 (Springer (New York), 1999).
Hope, A.C.A. A simplified Monte-Carlo significance test procedure. J. R. Stat. Soc. Ser. B, 582–598 (1968).
Acknowledgements
We thank M. Vinson, C. Okoye, J. Joffee-Block and P. Otto for technical assistance, and L. Jacobson for discussions of the data. The project was funded by the National Cancer Institute (R01-HD37356 to S.W.), the National Institute of Allergy and Infectious Diseases (P30-CA79458 to S.W.) and the National Institutes of Health (U01-AI-35039 to J.P. and S.W.). Additional support was provided by the Elizabeth Glazer Pediatric AIDS Foundation (R.F. and B.K.), a Los Alamos National Laboratory Program Developmental Award (B.K., M.F. and J.T.), the My Brother Joey Foundation (E.T.) and an anonymous foundation (S.W.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Trachtenberg, E., Korber, B., Sollars, C. et al. Advantage of rare HLA supertype in HIV disease progression. Nat Med 9, 928–935 (2003). https://doi.org/10.1038/nm893
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm893