Abstract
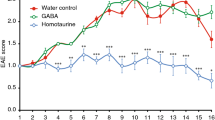
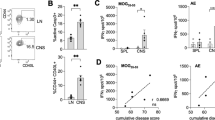
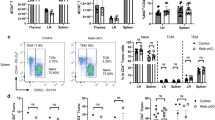
Multiple sclerosis (MS) is a devastating neuroinflammatory disorder of the central nervous system (CNS) in which T cells that are reactive with major components of myelin sheaths have a central role. The receptor for advanced glycation end products (RAGE) is present on T cells, mononuclear phagocytes and endothelium. Its pro-inflammatory ligands, S100-calgranulins, are upregulated in MS and in the related rodent model, experimental autoimmune encephalomyelitis (EAE). Blockade of RAGE suppressed EAE when disease was induced by myelin basic protein (MBP) peptide or encephalitogenic T cells, or when EAE occurred spontaneously in T-cell receptor (TCR)-transgenic mice devoid of endogenous TCR-α and TCR-β chains. Inhibition of RAGE markedly decreased infiltration of the CNS by immune and inflammatory cells. Transgenic mice with targeted overexpression of dominant-negative RAGE in CD4+ T cells were resistant to MBP-induced EAE. These data reinforce the importance of RAGE-ligand interactions in modulating properties of CD4+ T cells that infiltrate the CNS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bauer, J., Rauschka, H. & Lassmann, H. Inflammation in the nervous system: the human perspective. Glia 36, 235–243 (2001).
Hohlfeld, R. & Wekerle, H. Immunological update on multiple sclerosis. Curr. Opin. Neurol. 14, 299–304 (2001).
Zamvil, S. et al. T cell clones specific for myelin basic protein induce chronic relapsing paralysis and demyelination. Nature 317, 355–358 (1985).
Owens, T., Wekerle, H. & Antel, J. Genetic models for CNS inflammation. Nat. Med. 7, 161–166 (2001).
Schmidt, A.-M., Yan, S.-D., Yan, S.-F. & Stern, D.M. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J. Clin. Invest. 108, 949–955 (2001).
Hofmann, M. et al. RAGE mediates a novel proinflammatory axis: the cell surface receptor for S100/calgranulin polypeptides. Cell 97, 889–901 (1999).
Taguchi, A. et al. Blockade of RAGE/amphoterin suppresses tumor growth and metastases. Nature 405, 354–360 (2000).
Hori, O. et al. RAGE is a cellular binding site for amphoterin: mediation of neurite outgrowth and co-expression of RAGE and amphoterin in the developing nervous system. J. Biol. Chem. 270, 25752–25761 (1995).
Schafer, B. & Heizmann, C. The S100 family of EF-hand calcium-binding proteins: functions and pathology. TIBS 21, 134–140 (1996).
Wong, F., Dittel, B. & Janeway, C. Transgenes and knockout mutations in animal models of type 1 diabetes and multiple sclerosis. Immunol. Rev. 169, 93–106 (1999).
Baron, J., Madri, J., Ruddle, N., Hashim, G. & Janeway, C. Surface expression of α4-integrin by CD4 T cells is required for their entry into brain parenchyma. J. Exp. Med. 177, 57–68 (1993).
Park, L. et al. Suppression of accelerated diabetic atherosclerosis by sRAGE. Nat. Med. 4, 1025–1031 (1998).
Kaltschmidt, C. et al. Transcription factor NFκB is activated in microglia during EAE. J. Neuroimmunol. 55, 99–106 (1994).
Pahan, K. & Schmid, M. Activation of NFκB in the spinal cord of EAE. Neurosci. Lett. 287, 17–20 (2000).
Nygardas, P., Maatta, J. & Hinkkanen, A. Chemokine expression by CNS resident cells and infiltrating neutrophils during EAE in the Balb/c mouse. Eur. J. Immunol. 30, 1911–1918 (2000).
Karpus, W. & Ransohoff, R. Chemokine regulation of EAE: temporal and spatial expression patterns govern disease pathogenesis. J. Immunol. 161, 2667–2671 (1998).
Carlos, T. et al. VCAM-1 mediates lymphocyte adherence to cytokine-activated cultured human endothelial cells. Blood 76, 965–970 (1990).
Yednock, T. et al. Prevention of EAE by antibodies against α4β1-integrin. Nature 356, 63–66 (1992).
Graesser, D., Mahooti, S. & Madri, J. Distinct roles for MMP-2 and α4-integrin in autoimmune T cell extravasation and residency in brain parenchyma during EAE. J. Neuroimmunol. 109, 121–131 (2000).
Jiang, H., Braunstein, N., Yu, B., Winchester, R. & Chess, L. CD8 T cells control the Th phenotype of MBP-reactive CD4 T cells in EAE mice. Proc. Natl. Acad. Sci. USA 98, 6301–6306 (2001).
Hafler, D. & Weiner, H. Immunologic mechanisms and therapy in MS. Immunol. Rev. 144, 75–107 (1995).
Olivares-Villagomez, D., Wang, Y. & LaFaille, J. Regulatory CD4+ T cells expressing endogenous T cell receptor chains protect myelin basic protein-specific transgenic mice from spontaneous autoimmune encephalomyelitis. J. Exp. Med. 188, 1883–1894 (1998).
Lafaille, J., Nagashima, K., Katsuki, M. & Tonegawa, S. High incidence of spontaneous EAE in immunodeficient anti-MBP TCR transgenic mice. Cell 78, 399–408 (1994).
Adlam, M., Duncan, D., Ng, D. & Siu, G. Positive selection induces CD4 promoter and enhancer function. Int. Immunol. 9, 877–887 (1997).
Neuhaus, O., Farina, C., Wekerle, H. & Hohlfield, R. Mechanisms of action of glatiramer acetate in multiple sclerosis. Neurology 56, 702–708 (2001).
Farina, C. et al. Treatment of multiple sclerosis with Copaxone: Elispot assay detects IL-4 and interferon-γ response in blood cells. Brain 124 (pt 4), 705–719 (2001).
Howard, L. et al. Mechanisms of immunotherapeutic intervention by anti-CD40L antibody in an animal model of MS. J. Clin. Invest. 103, 281–290 (1999).
Girvin, A. et al. A critical role for B7/CD28 costimulation in EAE: a comparative study using costimulatory molecule-deficient mice and monoclonal antibody blockade. J. Immunol. 164, 136–143 (2000).
Zamvil, S. & Steinman, L. The T lymphocyte in EAE. Annu. Rev. Immunol. 8, 579–621 (1990).
Raine, C. The Dale E. McFarlin Memorial Lecture: the immunology of the MS lesion. Ann. Neurol. 36, S61–S72 (1994).
Brocke, S., Piercy, C., Steinman, L., Weissman, I. & Veroma, T. Antibodies to CD44 and integrin-α4, but not L-selectin, prevent CNS inflammation and EAE by blocking secondary leukocyte recruitment. Proc. Natl. Acad. Sci. USA 96, 6896–6901 (1999).
Kobayashi, Y. et al. Antibodies against LFA-1 and ICAM-1 together suppress the progression of EAE. Cell. Immunol. 46, 295–303 (1995).
Pitt, D., Werner, P. & Raine, C. Glutamate excitotoxicity in a model of MS. Nat. Med. 6, 67–70 (2000).
Smith, T., Groom, T., Zhu, B. & Turski, L. Autoimmune encephalomyelitis ameliorated by AMPA antagonists. Nat. Med. 6, 62–66 (2000).
Sousa, M. et al. FAP: RAGE-dependent triggering of neuronal inflammatory and apoptotic pathways. J. Neurosci. 21, 7576–7586 (2001).
Jiang, H., Zhang, S-L. & Pernis, B. Role of CD8+ T cells in murine EAE. Science 256, 1213–1215 (1992).
Lafaille, J. et al. MBP-specific Th2 cells cause EAE in immunodeficient hosts rather than protect them from disease. J. Exp. Med. 186, 307–312 (1997).
Yan, S.-D. et al. Nonenzymatically glycated tau in AD induces neuronal oxidant stress resulting in cytokine gene expression and release of Aβ. Nat. Med. 1, 693–699 (1995).
Acknowledgements
This work was supported by grants from the US Public Health Service (NS42855, AI44927, AI46132) and the Multiple Sclerosis Society (RG2938). We thank A.P. Hays for providing human MS samples and suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Yan, S., Wu, ZY., Zhang, H. et al. Suppression of experimental autoimmune encephalomyelitis by selective blockade of encephalitogenic T-cell infiltration of the central nervous system. Nat Med 9, 287–293 (2003). https://doi.org/10.1038/nm831
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm831