Abstract
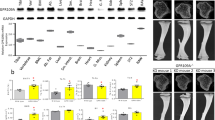
Osteoclasts are bone-resorbing cells derived from hematopoietic precursors of the monocyte-macrophage lineage. Regulation of osteoclast function is central to the understanding of bone diseases such as osteoporosis, rheumatoid arthritis and osteopetrosis1. Although peroxisome proliferator–activated receptor-γ (PPAR-γ) has been shown to inhibit osteoblast differentiation2,3, its role, if any, in osteoclasts is unknown. This is a clinically crucial question because PPAR-γ agonists, “such as thiazolidinediones—” a class of insulin-sensitizing drugs, have been reported to cause a higher rate of fractures in human patients4,5. Here we have uncovered a pro-osteoclastogenic effect of PPAR-γ by using a Tie2Cre/flox mouse model in which PPAR-γ is deleted in osteoclasts but not in osteoblasts. These mice develop osteopetrosis characterized by increased bone mass, reduced medullary cavity space and extramedullary hematopoiesis in the spleen. These defects are the result of impaired osteoclast differentiation and compromised receptor activator of nuclear factor-κB ligand signaling and can be rescued by bone marrow transplantation. Moreover, ligand activation of PPAR-γ by rosiglitazone exacerbates osteoclast differentiation in a receptor-dependent manner. Our examination of the underlying mechanisms suggested that PPAR-γ functions as a direct regulator of c-fos expression, an essential mediator of osteoclastogenesis6. Therefore, PPAR-γ and its ligands have a previously unrecognized role in promoting osteoclast differentiation and bone resorption.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tolar, J., Teitelbaum, S.L. & Orchard, P.J. Osteopetrosis. N. Engl. J. Med. 351, 2839–2849 (2004).
Akune, T. et al. PPAR γ insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J. Clin. Invest. 113, 846–855 (2004).
Cock, T.A. et al. Enhanced bone formation in lipodystrophic PPARγhyp/hyp mice relocates haematopoiesis to the spleen. EMBO Rep. 5, 1007–1012 (2004).
Grey, A. Skeletal consequences of thiazolidinedione therapy. Osteoporos. Int. published online, doi:10.1007/s00198-007-0477-y (28 September 2007).
Kahn, S.E. et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N. Engl. J. Med. 355, 2427–2443 (2006).
Grigoriadis, A.E. et al. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science 266, 443–448 (1994).
Barak, Y. et al. PPAR γ is required for placental, cardiac, and adipose tissue development. Mol. Cell 4, 585–595 (1999).
Kubota, N. et al. PPAR γ mediates high-fat diet–induced adipocyte hypertrophy and insulin resistance. Mol. Cell 4, 597–609 (1999).
Rosen, E.D. et al. PPAR γ is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell 4, 611–617 (1999).
Ali, A.A. et al. Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology 146, 1226–1235 (2005).
Li, M. et al. Surface-specific effects of a PPARγ agonist, darglitazone, on bone in mice. Bone 39, 796–806 (2006).
Rzonca, S.O., Suva, L.J., Gaddy, D., Montague, D.C. & Lecka-Czernik, B. Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology 145, 401–406 (2004).
Sottile, V., Seuwen, K. & Kneissel, M. Enhanced marrow adipogenesis and bone resorption in estrogen-deprived rats treated with the PPARgamma agonist BRL49653 (rosiglitazone). Calcif. Tissue Int. 75, 329–337 (2004).
Kisanuki, Y.Y. et al. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev. Biol. 230, 230–242 (2001).
He, W. et al. Adipose-specific peroxisome proliferator–activated receptor γ knockout causes insulin resistance in fat and liver but not in muscle. Proc. Natl. Acad. Sci. USA 100, 15712–15717 (2003).
Constien, R. et al. Characterization of a novel EGFP reporter mouse to monitor Cre recombination as demonstrated by a Tie2 Cre mouse line. Genesis 30, 36–44 (2001).
Wan, Y. et al. Maternal PPAR γ protects nursing neonates by suppressing the production of inflammatory milk. Genes Dev. 21, 1895–1908 (2007).
Takakura, N. et al. Critical role of the TIE2 endothelial cell receptor in the development of definitive hematopoiesis. Immunity 9, 677–686 (1998).
Cantor, A.B. & Orkin, S.H. Transcriptional regulation of erythropoiesis: an affair involving multiple partners. Oncogene 21, 3368–3376 (2002).
Taichman, R.S. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem cell niche. Blood 105, 2631–2639 (2005).
Kawano, H. et al. Suppressive function of androgen receptor in bone resorption. Proc. Natl. Acad. Sci. USA 100, 9416–9421 (2003).
Karsenty, G. & Wagner, E.F. Reaching a genetic and molecular understanding of skeletal development. Dev. Cell 2, 389–406 (2002).
Teitelbaum, S.L. Bone resorption by osteoclasts. Science 289, 1504–1508 (2000).
Wada, T., Nakashima, T., Hiroshi, N. & Penninger, J.M. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol. Med. 12, 17–25 (2006).
Matsuo, K. et al. Fosl1 is a transcriptional target of c-Fos during osteoclast differentiation. Nat. Genet. 24, 184–187 (2000).
Ricote, M., Li, A.C., Willson, T.M., Kelly, C.J. & Glass, C.K. The peroxisome proliferator–activated receptor-γ is a negative regulator of macrophage activation. Nature 391, 79–82 (1998).
Leesnitzer, L.M. et al. Functional consequences of cysteine modification in the ligand binding sites of peroxisome proliferator activated receptors by GW9662. Biochemistry 41, 6640–6650 (2002).
Lee, G. et al. T0070907, a selective ligand for peroxisome proliferator-activated receptor γ, functions as an antagonist of biochemical and cellular activities. J. Biol. Chem. 277, 19649–19657 (2002).
Grey, A. et al. The peroxisome proliferator-activated receptor-γ agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women: a randomized, controlled trial. J. Clin. Endocrinol. Metab. 92, 1305–1310 (2007).
Chui, P.C., Guan, H.P., Lehrke, M. & Lazar, M.A. PPARγ regulates adipocyte cholesterol metabolism via oxidized LDL receptor 1. J. Clin. Invest. 115, 2244–2256 (2005).
Acknowledgements
We would like to thank the Department of Radiology and Moores Cancer Center at the University of California at San Diego for the bone analyses, M. Yanagisawa (University of Texas Southwestern Medical Center) for providing the Tie2Cre transgenic mice, I. Verma (Salk Institute) for providing the Ub-GFP transgenic mice, S. Hong, J. Jonker, R. Yu and Y. Wan for discussion and critical reading of the manuscript, and L. Ong and S. Ganley for administrative assistance. R.E. is an investigator of the Howard Hughes Medical Institute at the Salk Institute and the March of Dimes Chair in Molecular and Developmental Biology. Y.W. is supported by a postdoctoral fellowship from the American Cancer Society (PF-03-081-01-TBE). This work was supported by the Howard Hughes Medical Institute and the National Institutes of Health (SCOR/HL569898, HL07770 and DK57978).
Author information
Authors and Affiliations
Contributions
Y.W. conceived the project, designed and performed most of the experiments and wrote the manuscript and L.-W.C. performed the experiments by assisting with tissue collection, DNA, RNA and protein isolation, and analyses. R.M.E. supervised the study and edited the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–7 and Supplementary Discussion (PDF 570 kb)
Rights and permissions
About this article
Cite this article
Wan, Y., Chong, LW. & Evans, R. PPAR-γ regulates osteoclastogenesis in mice. Nat Med 13, 1496–1503 (2007). https://doi.org/10.1038/nm1672
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1672