Abstract
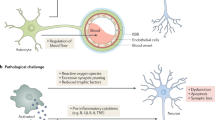
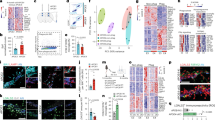
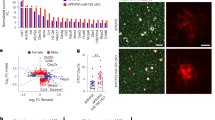
Microglia are the principal immune cells of the brain. In Alzheimer disease, these brain mononuclear phagocytes are recruited from the blood and accumulate in senile plaques. However, the role of microglia in Alzheimer disease has not been resolved. Microglia may be neuroprotective by phagocytosing amyloid-β (Aβ), but their activation and the secretion of neurotoxins may also cause neurodegeneration. Ccr2 is a chemokine receptor expressed on microglia, which mediates the accumulation of mononuclear phagocytes at sites of inflammation. Here we show that Ccr2 deficiency accelerates early disease progression and markedly impairs microglial accumulation in a transgenic mouse model of Alzheimer disease (Tg2576). Alzheimer disease mice deficient in Ccr2 accumulated Aβ earlier and died prematurely, in a manner that correlated with Ccr2 gene dosage, indicating that absence of early microglial accumulation leads to decreased Aβ clearance and increased mortality. Thus, Ccr2-dependent microglial accumulation plays a protective role in the early stages of Alzheimer disease by promoting Aβ clearance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Perry, V.H. & Gordon, S. Macrophages and microglia in the nervous system. Trends Neurosci. 11, 273–277 (1988).
Lawson, L.J., Perry, V.H. & Gordon, S. Turnover of resident microglia in the normal adult mouse brain. Neuroscience 48, 405–415 (1992).
Malm, T.M. et al. Bone-marrow-derived cells contribute to the recruitment of microglial cells in response to beta-amyloid deposition in APP/PS1 double transgenic Alzheimer mice. Neurobiol. Dis. 18, 134–142 (2005).
Simard, A.R., Soulet, D., Gowing, G., Julien, J.P. & Rivest, S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer's disease. Neuron 49, 489–502 (2006).
Perlmutter, L.S., Scott, S.A., Barron, E. & Chui, H.C. MHC class II-positive microglia in human brain: association with Alzheimer lesions. J. Neurosci. Res. 33, 549–558 (1992).
McGeer, P.L., Itagaki, S., Tago, H. & McGeer, E.G. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci. Lett. 79, 195–200 (1987).
Stalder, A.K. et al. Invasion of hematopoietic cells into the brain of amyloid precursor protein transgenic mice. J. Neurosci. 25, 11125–11132 (2005).
Stalder, M. et al. Association of microglia with amyloid plaques in brains of APP23 transgenic mice. Am. J. Pathol. 154, 1673–1684 (1999).
Qiu, W.Q. et al. Insulin-degrading enzyme regulates extracellular levels of amyloid beta-protein by degradation. J. Biol. Chem. 273, 32730–32738 (1998).
Qiu, W.Q., Ye, Z., Kholodenko, D., Seubert, P. & Selkoe, D.J. Degradation of amyloid beta-protein by a metalloprotease secreted by microglia and other neural and non-neural cells. J. Biol. Chem. 272, 6641–6646 (1997).
Weldon, D.T. et al. Fibrillar beta-amyloid induces microglial phagocytosis, expression of inducible nitric oxide synthase, and loss of a select population of neurons in the rat CNS in vivo. J. Neurosci. 18, 2161–2173 (1998).
Koenigsknecht, J. & Landreth, G. Microglial phagocytosis of fibrillar beta-amyloid through a beta1 integrin-dependent mechanism. J. Neurosci. 24, 9838–9846 (2004).
Paresce, D.M., Ghosh, R.N. & Maxfield, F.R. Microglial cells internalize aggregates of the Alzheimer's disease amyloid beta-protein via a scavenger receptor. Neuron 17, 553–565 (1996).
El Khoury, J. et al. Scavenger receptor-mediated adhesion of microglia to beta-amyloid fibrils. Nature 382, 716–719 (1996).
Yan, S.D. et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature 382, 685–691 (1996).
ElKhoury, J., Hickman, S.E., Thomas, C.A., Loike, J.D. & Silverstein, S.C. Microglia, scavenger receptors, and the pathogenesis of Alzheimerz's disease. Neurobiol. Aging. 19, 81–84 (1998).
El Khoury, J.B. et al. CD36 mediates the innate host response to beta-amyloid. J. Exp. Med. 197, 1657–1666 (2003).
Luster, A.D. Chemokines–chemotactic cytokines that mediate inflammation. N. Engl. J. Med. 338, 436–445 (1998).
Charo, I.F. & Ransohoff, R.M. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 354, 610–621 (2006).
Boddeke, E.W. et al. Cultured rat microglia express functional beta-chemokine receptors. J. Neuroimmunol. 98, 176–184 (1999).
Izikson, L., Klein, R.S., Charo, I.F., Weiner, H.L. & Luster, A.D. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2. J. Exp. Med. 192, 1075–1080 (2000).
Babcock, A.A., Kuziel, W.A., Rivest, S. & Owens, T. Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J. Neurosci. 23, 7922–7930 (2003).
Charo, I.F. & Peters, W. Chemokine receptor 2 (CCR2) in atherosclerosis, infectious diseases, and regulation of T-cell polarization. Microcirculation 10, 259–264 (2003).
Ishizuka, K. et al. Identification of monocyte chemoattractant protein-1 in senile plaques and reactive microglia of Alzheimer's disease. Psychiatry Clin. Neurosci. 51, 135–138 (1997).
Smits, H.A. et al. Amyloid-beta-induced chemokine production in primary human macrophages and astrocytes. J. Neuroimmunol. 127, 160–168 (2002).
Hsiao, K. et al. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science 274, 99–102 (1996).
Frautschy, S.A. et al. Microglial response to amyloid plaques in APPsw transgenic mice. Am. J. Pathol. 152, 307–317 (1998).
Benzing, W.C. et al. Evidence for glial-mediated inflammation in aged APP(SW) transgenic mice. Neurobiol. Aging 20, 581–589 (1999).
Leissring, M.A. et al. Enhanced proteolysis of beta-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron 40, 1087–1093 (2003).
Robbins, E.M. et al. Kinetics of cerebral amyloid angiopathy progression in a transgenic mouse model of Alzheimer disease. J. Neurosci. 26, 365–371 (2006).
Miao, J. et al. Cerebral microvascular amyloid beta protein deposition induces vascular degeneration and neuroinflammation in transgenic mice expressing human vasculotropic mutant amyloid beta precursor protein. Am. J. Pathol. 167, 505–515 (2005).
Winkler, D.T. et al. Spontaneous hemorrhagic stroke in a mouse model of cerebral amyloid angiopathy. J. Neurosci. 21, 1619–1627 (2001).
Kimberly, W.T. et al. Gamma-secretase is a membrane protein complex comprised of presenilin, nicastrin, Aph-1, and Pen-2. Proc. Natl. Acad. Sci. USA 100, 6382–6387 (2003).
Haass, C. Take five–BACE and the gamma-secretase quartet conduct Alzheimer's amyloid beta-peptide generation. EMBO J. 23, 483–488 (2004).
Kuo, Y.M. et al. Elevated A beta and apolipoprotein E in A betaPP transgenic mice and its relationship to amyloid accumulation in Alzheimer's disease. Mol. Med. 6, 430–439 (2000).
Fryer, J.D. et al. Apolipoprotein E markedly facilitates age-dependent cerebral amyloid angiopathy and spontaneous hemorrhage in amyloid precursor protein transgenic mice. J. Neurosci. 23, 7889–7896 (2003).
Iwata, N. et al. Metabolic regulation of brain Abeta by neprilysin. Science 292, 1550–1552 (2001).
Bard, F. et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat. Med. 6, 916–919 (2000).
Boring, L. et al. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C–C chemokine receptor 2 knockout mice. J. Clin. Invest. 100, 2552–2561 (1997).
Janelsins, M.C. et al. Early correlation of microglial activation with enhanced Tumor necrosis factor-alpha and monocyte chemoattractant protein-1 expression specifically within the entorhinal cortex of triple transgenic Alzheimer's disease mice. J. Neuroinflammation 2, 23 (2005).
Yamamoto, M. et al. Overexpression of monocyte chemotactic protein-1/CCL2 in beta-amyloid precursor protein transgenic mice show accelerated diffuse beta-amyloid deposition. Am. J. Pathol. 166, 1475–1485 (2005).
Huang, D. et al. Chronic expression of monocyte chemoattractant protein-1 in the central nervous system causes delayed encephalopathy and impaired microglial function in mice. FASEB J. 19, 761–772 (2005).
Meda, L. et al. Beta-amyloid (25–35) peptide and IFN-gamma synergistically induce the production of the chemotactic cytokine MCP-1/JE in monocytes and microglial cells. J. Immunol. 157, 1213–1218 (1996).
Sedgwick, J.D. et al. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc. Natl. Acad. Sci. USA 88, 7438–7442 (1991).
Eugenin, E.A. et al. Microglia at brain stab wounds express connexin 43 and in vitro form functional gap junctions after treatment with interferon-gamma and tumor necrosis factor-alpha. Proc. Natl. Acad. Sci. USA 98, 4190–4195 (2001).
Bennett, J.L. et al. CCL2 transgene expression in the central nervous system directs diffuse infiltration of CD45(high)CD11b(+) monocytes and enhanced Theiler's murine encephalomyelitis virus-induced demyelinating disease. J. Neurovirol. 9, 623–636 (2003).
Coraci, I.S. et al. CD36, a class B scavenger receptor, is expressed on microglia in Alzheimer's disease brains and can mediate production of reactive oxygen species in response to beta-amyloid fibrils. Am. J. Pathol. 160, 101–112 (2002).
Walsh, D.M. et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416, 535–539 (2002).
Ghiso, J. & Frangione, B. Cerebral amyloidosis, amyloid angiopathy, and their relationship to stroke and dementia. J. Alzheimers Dis. 3, 65–73 (2001).
Wyss-Coray, T. et al. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat. Med. 9, 453–457 (2003).
Acknowledgements
We thank D. Selkoe for discussing the data and for suggestions, K. Hsiao-Ashe for permission to use the APP mice, I. Charo (Gladstone Institute of Cardiovascular Disease) for providing the Ccr2−/− mice, the Harvard Center for Neurodegeneration and Repair for funding J.E.K. and C.G., the American Health Assistance Foundation and the Dana Foundation for funding J.E.K. and the US National Institutes of Health for funding J.E.K. and A.D.L.
Author information
Authors and Affiliations
Contributions
J.E.K. designed the experiments, analyzed the data, wrote the manuscript, performed the chemotaxis experiments and directly supervised M.T. in breeding the mice and in performing the immunostaining and the ELISA experiments. S.E.H. isolated brain cells and macrophages and performed the Aβ binding experiments, and the laser capture microdissection experiments. T.K.M. performed the Q-PCR. S.E.H. and T.K.M. were also involved in detailed discussions of the data and manuscript. K.T. and C.G. designed and performed the stereotaxic microinjection experiments. A.D.L. participated in designing experiments, evaluating data and writing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Florid Aβ deposition and senile plaque formation in aged APP mice. (PDF 72 kb)
Supplementary Fig. 2
Ccl2 is produced in Cd11b-positive cells in APP mice but not in Gfap-positive cells. (PDF 766 kb)
Supplementary Fig. 3
Expression of Ccr2 correlates with Cd11b expression in brain cells. (PDF 331 kb)
Supplementary Fig. 4
Absence of microglial accumulation in 65-day old Ccr2 deficient APP mice. (PDF 73 kb)
Supplementary Fig. 5
Migration of resident peritoneal macrophages from Ccr2+/−, Ccr2−/−, and WT mice to Ccl2 and Ccl3. (PDF 75 kb)
Supplementary Table 1
Sequences of primers used for Q-PCR. (PDF 51 kb)
Rights and permissions
About this article
Cite this article
El Khoury, J., Toft, M., Hickman, S. et al. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med 13, 432–438 (2007). https://doi.org/10.1038/nm1555
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1555
This article is cited by
-
Peripheral blood amyloid-β involved in the pathogenesis of Alzheimer’s disease via impacting on peripheral innate immune cells
Journal of Neuroinflammation (2024)
-
Experimental colitis in young Tg2576 mice accelerates the onset of an Alzheimer’s-like clinical phenotype
Alzheimer's Research & Therapy (2024)
-
Monocyte-derived cells invade brain parenchyma and amyloid plaques in human Alzheimer’s disease hippocampus
Acta Neuropathologica Communications (2023)
-
Blood and CSF chemokines in Alzheimer’s disease and mild cognitive impairment: a systematic review and meta-analysis
Alzheimer's Research & Therapy (2023)
-
Mechanisms of myeloid cell entry to the healthy and diseased central nervous system
Nature Immunology (2023)