Abstract
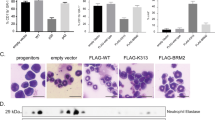
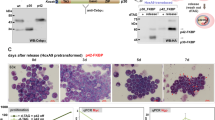
We demonstrate here that lymphoid enhancer-binding factor 1 (LEF-1) mediates the proliferation, survival and differentiation of granulocyte progenitor cells. We initially documented the importance of this transcription factor in the bone marrow of individuals with severe congenital neutropenia (CN) with a 'differentiation block' at the promyelocytic stage of myelopoiesis1. LEF-1 expression was greatly reduced or even absent in CN arrested promyelocytes, resulting in defective expression of the LEF-1 target genes CCND1, MYC and BIRC5, encoding cyclin D1 (ref. 2), c-Myc3 and survivin4, respectively. In contrast, healthy individuals showed highest LEF-1 expression in promyelocytes. Reconstitution of LEF-1 in early hematopoietic progenitors of two individuals with CN corrected the defective myelopoiesis and resulted in the differentiation of these progenitors into mature granulocytes. Repression of endogenous LEF-1 by specific short hairpin RNA inhibited proliferation and induced apoptosis of CD34+ progenitors from healthy individuals and of cells from two myeloid lines (HL-60 and K562). C/EBPα, a key transcription factor in granulopoiesis5,6, was directly regulated by LEF-1. These observations indicate that LEF-1 is an instructive factor regulating neutrophilic granulopoiesis whose absence plays a critical role in the defective maturation program of myeloid progenitors in individuals with CN. NOTE: In the version of this article initially published, the DOI was incorrect. The correct DOI is 10.1038/nm1474. The error has been corrected in the HTML and PDF versions of the article.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
19 October 2006
In the version of this article initially published, the DOI was incorrect. The correct DOI is 10.1038/nm1474. The error has been corrected in the HTML and PDF versions of the article.
References
Welte, K., Zeidler, C. & Dale, D.C. Severe congenital neutropenia. Semin. Hematol. 43, 189–195 (2006).
Shtutman, M. et al. The cyclin D1 gene is a target of the β-catenin/LEF-1 pathway. Proc. Natl Acad. Sci. USA 96, 5522–5527 (1999).
He, T.C. et al. Identification of c-Myc as a target of the APC pathway. Science 281, 1509–1512 (1998).
Kim, P.J., Plescia, J., Clevers, H., Fearon, E.R. & Altieri, D.C. Survivin and molecular pathogenesis of colorectal cancer. Lancet 362, 205–209 (2003).
Radomska, H.S. et al. CCAAT/enhancer binding protein α is a regulatory switch sufficient for induction of granulocytic development from bipotential myeloid progenitors. Mol. Cell. Biol. 18, 4301–4314 (1998).
Zhang, P. et al. Induction of granulocytic differentiation by 2 pathways. Blood 99, 4406–4412 (2002).
Rosenberg, P.S. et al. The incidence of leukemia and mortality from sepsis in patients with severe congenital neutropenia receiving long-term G-CSF therapy. Blood 107, 4628–4635 (2006).
Konishi, N. et al. Defective proliferation of primitive myeloid progenitor cells in patients with severe congenital neutropenia. Blood 94, 4077–4083 (1999).
Cario, G. et al. Heterogeneous expression pattern of pro- and anti-apoptotic factors in myeloid progenitor cells of patients with severe congenital neutropenia treated with granulocyte colony-stimulating factor. Br. J. Haematol. 129, 275–278 (2005).
Dale, D.C. et al. Mutations in the gene encoding neutrophil elastase in congenital and cyclic neutropenia. Blood 96, 2317–2322 (2000).
Dong, F. et al. Mutations in the gene for the granulocyte colony-stimulating-factor receptor in patients with acute myeloid leukemia preceded by severe congenital neutropenia. N. Engl. J. Med. 333, 487–493 (1995).
Person, R.E. et al. Mutations in proto-oncogene GFI1 cause human neutropenia and target ELA2. Nat. Genet. 34, 308–312 (2003).
Devriendt, K. et al. Constitutively activating mutation in WASP causes X-linked severe congenital neutropenia. Nat. Genet. 27, 313–317 (2001).
van de Wetering, M., de Lau, W. & Clevers, H. WNT signaling and lymphocyte development. Cell 109 (Suppl.), 13–19 (2002).
Nawshad, A. & Hay, E.D. TGFβ3 signaling activates transcription of the LEF1 gene to induce epithelial mesenchymal transformation during mouse palate development. J. Cell Biol. 163, 1291–1301 (2003).
Ross, D.A. & Kadesch, T. The notch intracellular domain can function as a coactivator for LEF-1. Mol. Cell. Biol. 21, 7537–7544 (2001).
Merrill, B.J., Gat, U., DasGupta, R. & Fuchs, E. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev. 15, 1688–1705 (2001).
Hovanes, K. β-catenin–sensitive isoforms of lymphoid enhancer factor-1 are selectively expressed in colon cancer. Nat. Genet. 28, 53–57 (2001).
Giese, K., Kingsley, C., Kirshner, J.R. & Grosschedl, R. Assembly and function of a TCR alpha enhancer complex is dependent on LEF-1-induced DNA bending and multiple protein-protein interactions. Genes Dev. 9, 995–1008 (1995).
Travis, A., Amsterdam, A., Belanger, C. & Grosschedl, R. LEF-1, a gene encoding a lymphoid-specific protein with an HMG domain, regulates T-cell receptor α enhancer function. Genes Dev. 5, 880–894 (1991).
Hsu, S.-C., Galceran, J. & Grosschedl, R. Modulation of transcriptional regulation by LEF-1 in response to Wnt-1 signaling and association with β-catenin. Mol. Cell. Biol. 18, 4807–4818 (1998).
Reya, T. et al. Wnt signaling regulates B lymphocyte proliferation through a LEF-1 dependent mechanism. Immunity 13, 15–24 (2000).
Filali, M., Cheng, N., Abbott, D., Leontiev, V. & Engelhardt, J.F. Wnt-3A/β-catenin signaling induces transcription from the LEF-1 promoter. J. Biol. Chem. 277, 33398–33410 (2002).
Timchenko, N. et al. Autoregulation of the human C/EBPα gene by stimulation of upstream stimulatory factor binding. Mol. Cell. Biol. 19, 1192–1202 (1995).
Suh, H.C. et al. C/EBPα determines hematopoietic cell fate in multipotential progenitor cells by inhibiting erythroid differentiation and inducing myeloid differentiation. Blood 107, 4308–4316 (2006).
Ross, S.E. et al. Phosphorylation of C/EBPα inhibits granulopoiesis. Mol. Cell. Biol. 24, 675–686 (2004).
Cammenga, J. et al. Induction of C/EBPα activity alters gene expression and differentiation of human CD34+ cells. Blood 101, 2206–2214 (2003).
Zhang, D.E. et al. Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein α-deficient mice. Proc. Natl. Acad. Sci. USA 94, 569–574 (1997).
Pabst, T. et al. Dominant-negative mutations of CEBPA, encoding CCAAT/enhancer binding protein-alpha (C/EBPα), in acute myeloid leukemia. Nat. Genet. 27, 263–270 (2001).
Radomska, H.S. et al. Block of C/EBPα function by phosphorylation in acute myeloid leukemia with FLT3 activating mutations. J. Exp. Med. 203, 371–381 (2006).
Welte, K. et al. Differential effects of granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor in children with severe congenital neutropenia. Blood 75, 1056–1063 (1990).
Harris, T.E., Albrecht, J.H., Nakanishi, M. & Darlington, G.J. CCAAT/enhancer-binding protein-α cooperates with p21 to inhibit cyclin-dependent kinase-2 activity and induces growth arrest independent of DNA binding. J. Biol. Chem. 276, 29200–29209 (2001).
Johansen, L.M. c-Myc is a critical target for c/EBPα in granulopoiesis. Mol. Cell. Biol. 21, 3789–3806 (2001).
Waterman, M.L. Lymphoid enhancer factor/T cell factor expression in colorectal cancer. Cancer Metastasis Rev. 23, 41–52 (2004).
Liu, F., van den Broek, O., Destree, O. & Hoppler, S. Distinct roles for Xenopus Tcf/Lef genes in mediating specific responses to Wnt/β-catenin signalling in mesoderm development. Development 132, 5375–5385 (2005).
Scherr, M., Battmer, K., Ganser, A. & Eder, M. Modulation of gene expression by lentiviral-mediated delivery of small interfering RNA. Cell Cycle 2, 251–257 (2003).
Acknowledgements
We thank M. Morgan for critically reading the manuscript; V. Bucan and D. Lan for sequencing, G. Asgedom and A. Müller-Brechlin for excellent technical assistance, and M. Ballmaier and C. Reimer for assistance in cell sorting. We also thank the study subjects and their families for cooperation. This work was supported by the German Network “Congenital Bone Marrow Failure Syndromes” of the Federal Ministry of Education and Research (BMBF), Deutsche Forschungsgemeinschaft (SFB 566), Madeleine-Schickedanz-Kinderkrebsstiftung, and José Carreras Leukämie-Stiftung e.V.
Author information
Authors and Affiliations
Contributions
K.W., G.C., J.S. and M.St. made initial observations. J.S. designed and performed the main experiments, analyzed the data and wrote the manuscript. M.U. performed western blot analysis and wrote the manuscript. M.Sch., M.E. and K.B. designed shRNA constructs and transduced cells. A.S. and C.B. designed lv constructs that contained full-length LEF-1 or dnLEF-1 and produced viral supernatants. M.G. performed sequence analysis of ELA2 gene. C.Z. provided patients data. U.L. provided laser-assisted microscope. K.W. supervised experimentation, coordinated the project and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
mRNA expression of secretory granule proteins; LEF-1 mRNA/protein expression as well as TCF-3,-4 mRNA expression. (PDF 220 kb)
Supplementary Fig. 2
mRNA expression of indicated genes in sorted GFP− CD34+ CN cells from the LEF-1 lv and dnLEF-1 lv experiments. (PDF 26 kb)
Supplementary Fig. 3
mRNA expression of indicated genes in sorted RFP− cells from the shRNA transduction experiments. Morphology of LEF-1 and β-catenin shRNA transduced cells. (PDF 65 kb)
Supplementary Fig. 4
Effects of LEF-1 inhibition in two myeloid cell lines HL-60 and K562. (PDF 160 kb)
Supplementary Fig. 5
mRNA expression of indicated genes in sorted RFP− cells from the shRNA transduction experiments in K562 and HL-60 cell lines. (PDF 26 kb)
Supplementary Fig. 6
mRNA expression of indicated genes in sorted GFP− cells from the experiments in LEF-1 lv transduced CD34+ cells of healthy controls. (PDF 23 kb)
Supplementary Table 1
CN patients' characteristics and ELA2 mutations status. (PDF 15 kb)
Supplementary Table 2
Oligonucleotide sequences used for NoShift assay. (PDF 12 kb)
Rights and permissions
About this article
Cite this article
Skokowa, J., Cario, G., Uenalan, M. et al. LEF-1 is crucial for neutrophil granulocytopoiesis and its expression is severely reduced in congenital neutropenia. Nat Med 12, 1191–1197 (2006). https://doi.org/10.1038/nm1474
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1474