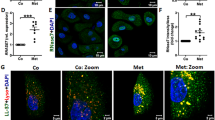
Abstract
The urinary tract functions in close proximity to the outside environment, yet must remain free of microbial colonization to avoid disease. The mechanisms for establishing an antimicrobial barrier in this area are not completely understood. Here, we describe the production and function of the cathelicidin antimicrobial peptides LL-37, its precursor hCAP-18 and its ortholog CRAMP in epithelial cells of human and mouse urinary tract, respectively. Bacterial contact with epithelial cells resulted in rapid production and secretion of the respective peptides, and in humans LL-37/hCAP-18 was released into urine. Epithelium-derived cathelicidin substantially contributed to the protection of the urinary tract against infection, as shown using CRAMP-deficient and neutrophil-depleted mice. In addition, clinical E. coli strains that were more resistant to LL-37 caused more severe urinary tract infections than did susceptible strains. Thus, cathelicidin seems to be a key factor in mucosal immunity of the urinary tract.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 415, 389–395 (2002).
Ganz, T. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3, 710–720 (2003).
Zanetti, M. Cathelicidins, multifunctional peptides of the innate immunity. J. Leukoc. Biol. 75, 39–48 (2004).
Nizet, V. et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 414, 454–457 (2001).
Moser, C. et al. β-Defensin 1 contributes to pulmonary innate immunity in mice. Infect. Immun. 70, 3068–3072 (2002).
Valore, E.V. et al. Human β-defensin-1: an antimicrobial peptide of urogenital tissues. J. Clin. Invest. 101, 1633–1642 (1998).
Morrison, G., Kilanowski, F., Davidson, D. & Dorin, J. Characterization of the mouse beta defensin 1, Defb1, mutant mouse model. Infect. Immun. 70, 3053–3060 (2002).
Zucht, H.D. et al. Human β-defensin-1: A urinary peptide present in variant molecular forms and its putative functional implication. Eur. J. Med. Res. 3, 315–323 (1998).
Zaiou, M. & Gallo, R.L. Cathelicidins, essential gene-encoded mammalian antibiotics. J. Mol. Med. 80, 549–561 (2002).
Tollin, M. et al. Antimicrobial peptides in the first line defence of human colon mucosa. Peptides 24, 523–530 (2003).
Agerberth, B. et al. Antibacterial components in bronchoalveolar lavage fluid from healthy individuals and sarcoidosis patients. Am. J. Respir. Crit. Care Med. 160, 283–290 (1999).
Gudmundsson, G.H. et al. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur. J. Biochem. 238, 325–332 (1996).
Malm, J. et al. The human cationic antimicrobial protein (hCAP-18) is expressed in the epithelium of human epididymis, is present in seminal plasma at high concentrations, and is attached to spermatozoa. Infect. Immun. 68, 4297–4302 (2000).
Gallo, R.L. et al. Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. J. Biol. Chem. 272, 13088–13093 (1997).
Sorensen, O.E. et al. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood 97, 3951–3959 (2001).
Zaiou, M., Nizet, V. & Gallo, R.L. Antimicrobial and protease inhibitory functions of the human cathelicidin (hCAP18/LL-37) prosequence. J. Invest. Dermatol. 120, 810–816 (2003).
Pestonjamasp, V.K., Huttner, K.H. & Gallo, R.L. Processing site and gene structure for the murine antimicrobial peptide CRAMP. Peptides 22, 1643–1650 (2001).
Brogden, K.A. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 3, 238–250 (2005).
Scott, M.G., Davidson, D.J., Gold, M.R., Bowdish, D. & Hancock, R.E. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J. Immunol. 169, 3883–3891 (2002).
Yang, D., Biragyn, A., Kwak, L.W. & Oppenheim, J.J. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol. 23, 291–296 (2002).
Bowdish, D.M., Davidson, D.J. & Hancock, R.E. A re-evaluation of the role of host defence peptides in mammalian immunity. Curr. Protein Pept. Sci. 6, 35–51 (2005).
Turner, J., Cho, Y., Dinh, N.N., Waring, A.J. & Lehrer, R.I. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob. Agents Chemother. 42, 2206–2214 (1998).
Faurschou, M. & Borregaard, N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 5, 1317–1327 (2003).
Hase, K., Eckmann, L., Leopard, J.D., Varki, N. & Kagnoff, M.F. Cell differentiation is a key determinant of cathelicidin LL-37/human cationic antimicrobial protein 18 expression by human colon epithelium. Infect. Immun. 70, 953–963 (2002).
Norden, C.W., Green, G.M. & Kass, E.H. Antibacterial mechanisms of the urinary bladder. J. Clin. Invest. 47, 2689–2700 (1968).
Justice, S.S. et al. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc. Natl. Acad. Sci. USA 101, 1333–1338 (2004).
Gordon, D.M. & Riley, M.A. A theoretical and experimental analysis of bacterial growth in the bladder. Mol. Microbiol. 6, 555–562 (1992).
Cox, C.E. & Hinman, F., Jr. Experiments with induced bacteriuria, vesical emptying and bacterial growth on the mechanism of bladder defense to infection. J. Urol. 86, 739–748 (1961).
Mulvey, M.A. et al. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282, 1494–1497 (1998).
Bates, J.M. et al. Tamm-Horsfall protein knockout mice are more prone to urinary tract infection: rapid communication. Kidney Int. 65, 791–797 (2004).
Fliedner, M., Mehls, O., Rauterberg, E.W. & Ritz, E. Urinary sIgA in children with urinary tract infection. J. Pediatr. 109, 416–421 (1986).
Abrink, M., Larsson, E., Gobl, A. & Hellman, L. Expression of lactoferrin in the kidney: implications for innate immunity and iron metabolism. Kidney Int. 57, 2004–2010 (2000).
Islam, D. et al. Downregulation of bactericidal peptides in enteric infections: a novel immune escape mechanism with bacterial DNA as a potential regulator. Nat. Med. 7, 180–185 (2001).
Bergman, P. et al. Neisseria gonorrhoeae downregulates expression of the human antimicrobial peptide LL-37. Cell. Microbiol. 7, 1009–1017 (2005).
Johansson, J., Gudmundsson, G.H., Rottenberg, M.E., Berndt, K.D. & Agerberth, B. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J. Biol. Chem. 273, 3718–3724 (1998).
Sorensen, O., Arnljots, K., Cowland, J.B., Bainton, D.F. & Borregaard, N. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood 90, 2796–2803 (1997).
Dorschner, R.A. et al. The mammalian ionic environment dictates microbial susceptibility to antimicrobial defense peptides. FASEB J. 20, 35–42 (2006).
Yan, H. & Hancock, R.E. Synergistic interactions between mammalian antimicrobial defense peptides. Antimicrob. Agents Chemother. 45, 1558–1560 (2001).
Braff, M.H., Zaiou, M., Fierer, J., Nizet, V. & Gallo, R.L. Keratinocyte production of cathelicidin provides direct activity against bacterial skin pathogens. Infect. Immun. 73, 6771–6781 (2005).
Yoshio, H. et al. Antimicrobial polypeptides of human vernix caseosa and amniotic fluid: implications for newborn innate defense. Pediatr. Res. 53, 211–216 (2003).
Todd, J.H., McMartin, K.E. & Sens, D.A. Enzymatic isolation and serum-free culture of human renal cells. in Human cell culture protocols (ed. Jones, G.E.) 431–436 (Humana Press, Totowa, 1996).
Rossi, M.R. et al. The immortalized UROtsa cell line as a potential cell culture model of human urothelium. Environ. Health Perspect. 109, 801–808 (2001).
Khalil, A. et al. Cytokine gene expression during experimental Escherichia coli pyelonephritis in mice. J. Urol. 158, 1576–1580 (1997).
Chromek, M. et al. Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinases-1 in acute pyelonephritis and renal scarring. Pediatr. Res. 53, 698–705 (2003).
Black, C.A. et al. Acute neutropenia decreases inflammation associated with murine vaginal candidiasis but has no effect on the course of infection. Infect. Immun. 66, 1273–1275 (1998).
Acknowledgements
We thank Z. Fehervízyová and T. Baltesová for help collecting urine samples, M. Lindh for technical assistance and G. Kronvall for help with single-strain regression analysis. This work was supported by ALF Project Funding, The Swedish Society of Medicine, The Swedish Association of Kidney Patients, funds from the Karolinska Institute, Magn. Bergvalls Foundation, Capio Foundation, The Swedish Research Council (04X-2887, 06X-11217), The Swedish Foundation for International Cooperation in Research and Higher Education (STINT), and the Knut and Alice Wallenberg Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
LL-37/hCAP-18 levels in urine of 28 healthy children and in 29 children with acute urinary tract infection. (PDF 77 kb)
Supplementary Fig. 2
Human LL-37/hCAP-18 mRNA and protein levels in noninfected renal and uroepithelial cells and tissues. (PDF 27 kb)
Rights and permissions
About this article
Cite this article
Chromek, M., Slamová, Z., Bergman, P. et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med 12, 636–641 (2006). https://doi.org/10.1038/nm1407
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1407