Abstract
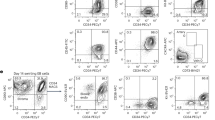
The development of stem–cell gene therapy is hindered by the absence of repopulation assays for primitive human hematopoietic cells. Current methods of gene transfer rely on in vitro colony–forming cell (CFC) and long–term culture–initiating cell (LTC–IC) assays, as well as inference from other mammalian species. We have identified a novel human hematopoietic cell, the SCID–repopulating cell (SRC), a cell more primitive than most LTC–ICs and CFCs. The SRC, exclusively present in the CD4+ CD8− fraction, is capable of multilineage repopulation of the bone marrow of nonobese diabetic mice with severe combined immunodeficiency disease (NOD/SCID mice). SRCs were rarely transduced with retroviruses, distinguishing them from most CFCs and LTC–ICs. This observation is consistent with the low level of gene marking seen in human gene therapy trials. An SRC assay may aid in the characterization of hematopoiesis, as well as the improvement of transduction methods.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Anderson, W.F. Human gene therapy. Science 256, 808–813 (1992).
Dick, J.E., Magli, M.C., Huszar, D., Phillips, R.A. & Bernstein, A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell 42, 71–79 (1985).
Schuening, F. et al. Retrovirus-mediated gene transduction into long-term re-populating marrow cells of the dog. Blood 78, 2568–2576 (1991).
Bodine, D.M. et al. Long-term in vivo expression of a murine adenosine deaminase gene in rhesus monkey hematopoietic cells of multiple lineages after retroviral mediated gene transfer into CD34+ bone marrow cells. Blood 82, 1975–1980 (1993).
Brenner, M.K. et al. Gene marking to determine whether autologous marrow infusion restores long-term haemopoiesis in cancer patients. Lancet 342, 1134–1137 (1993).
Deisseroth, A.B. et al. Genetic marking shows that Ph+ cells present in autologous transplants of chronic myelogenous leukemia (CML) contribute to relapse after autologous bone marrow in CML. Blood 83, 3068–3076 (1994).
Kohn, D. et al. Engraftment of gene-modified umbilical cord blood cells in neonates with adenosine deaminase deficiency. Nature Med. 1, 1017–1023 (1995).
Dunbar, C.E. et al. Retrovirally marked CD34-enriched peripheral blood and bone marrow cells contribute to long-term engraftment after autologous transplantation. Blood 85, 3048–3057 (1995).
Hock, R.A. & Miller, A.D. Retrovirus-mediated transfer and expression of drug resistance genes in human haematopoietic progenitor cells. Nature 320, 275–277 (1986).
Dick, J.E., Kamel-Reid, S., Murdoch, B. & Doedens, M. Gene transfer into normal human hematopoietic cells using in vitro and in vivo assays. Blood 78, 624–634 (1991).
Hughes, P.F., Eaves, C.J., Hogge, D.E. & Humphries, R.K. High-efficiency gene transfer to human hematopoietic cells maintained in long-term marrow culture. Blood 74, 1915–1922 (1989).
Eaves, C.J. et al. The human hematopoietic stem cell in vitro and in vivo. Blood Cells 18, 301–307 (1992).
Moritz, T., Patel, V.P. & Williams, D.A. Bone marrow extra cellular matrix molecules improve gene transfer into human hematopoietic cells via retroviral vectors. J. Clin. Invest. 93, 1451–1457 (1994).
Nolta, J.A., Hanley, M.B. & Kohn, D.B. Sustained human hematopoiesis in immunodeficient mice by cotransplantation of marrow stroma expressing human interleukin-3: Analysis of gene transduction of long-lived progenitors. Blood 83, 3041–3051 (1994).
Lapidot, T. et al. Cytokine stimulation of multilineage hematopoiesis from immature human cells engrafted in scid mice. Science 255, 1137–1141 (1992).
Vormoor, J. et al. Immature human cord blood progenitors engraft and proliferate to high levels in immune-deficient SCID mice. Blood 83, 2489–2497 (1994).
Baum, C.M., Weissman, I.L., Tsukamoto, A.S., Buckle, A.-S. & Peault, B. Isolation of a candidate human hematopoietic stem-cell population. Proc. Natl. Acad. Sci. USA 89, 2804–2808 (1992).
Shultz, L. et al. Multiple defects in innate and adaptive immunological function in NOD/LtSz-scid mice. J. Immunol 154, 180–191 (1995).
Cashman, J. et al. Proliferation and expansion of CD34+ human progenitor cells in the marrow of NOD/SCID mice. Blood 84, 368a (1994).
Larochelle, A. et al. Engraftment of immune-deficient mice with primitive hematopoietic cells from β-thalassemia and sickle cell anemia patients: Implications for evaluating human gene therapy protocols. Hum. Mol. Genet. 4, 163–172 (1995).
Vormoor, J., Lapidot, T., Larochelle, A. & Dick, J.E. Human hematopoiesis in SCID mice. in Human Hematopoiesis in SCID Mice (ed. Roncarolo, M.-G., Namikawa, R. & Péault, B.) 197–212 (Landes Co., Austin, Texas, 1995).
Dick, J. Normal and leukemic human stem cells assayed in SCID mice. Semin. Immunol. 8, 197–206 (1996).
Kimizuka, F. et al. Production and characterization of functional domains of human fibronectin fragment expressed in Escherichia coli . J. Biochem. 110, 284–291 (1991).
Bodine, D.M., McDonagh, K.T., Seidel, N.E. & Nienhuis, A.W. Survival and retrovirus infection of murine hematopoietic stem cells in vitro: Effects of 5-FU and method of infection. Exp. Hematol. 19, 206–212 (1991).
Moritz, T., Keller, D.C. & Williams, D.A. Human cord blood cells as targets fo gene transfer: Potential use in genetic therapy of severe combined immunodeficiency disease. J. Exp. Med. 178, 529 (1993).
Williams, D.A., Rios, M., Stephens, C. & Patel, V.P. Fibronectin and VLA-4 in haematopoietic stem cell-microenvironment interactions. Nature 352, 438–441 (1991).
Harrison, D.E., Lerner, C.P. & Spooncer, E. Erythropoietic repopulating ability of stem cells from long-term marrow culture. Blood 69, 1021–1025 (1987).
Ochman, H., Gerber, A.S. & Hartl, D.L. Genetic applications of an inverse polymerase chain reaction. Genetics 120, 621–623 (1988).
Civin, C. et al. Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J. Immunol. 133, 157–165 (1984).
Terstappen, L.W.W.M., Huang, S., Safford, M., Lansdorp, P.M. & Loken, M.R. Sequential generations of hematopoietic colonies derived from single nonlineage-committed CD34+CD38−progenitor cells. Blood 77, 1218–1227 (1991).
Hanenberg, H. et al. Colocalization of retrovirus and target cells on specific fibronectin fragments increases genetic transduction of mammalian cells. Nature Med. 2, 876–882 (1996).
Moritz, T. et al. Fibronectin improves transduction of reconstituting hematopoietic stem cells by retroviral vectors: Evidence for direct viral binding to chymotryptic carboxy-terminal fragments. Blood 88, 855–862 (1996).
Roath, S. et al. Specific capture of targeted hematopoietic cells by high gradient magnetic separation by the use of ordered wire array filters and tetrameric antibody complexes linked to a dextran iron particle. Prog. Clin. Biol. Res. 389, 155–163 (1994).
Huang, S. & Terstappen, L.W. Lymphoid and myeloid differentiation of single human CD34+, HLA-DR+, CD38-hematopoietic stem cells. Blood 83, 1515–1526 (1994).
Craig, W., Kay, R., Cutler, R.B. & Lansdorp, P.M. Expression of Thy-1 on human hematopoietic progenitor cells. J. Exp. Med. 177, 1331–1342 (1993).
Rusten, L.S. et al. Functional differences between CD38− and DR-subfractions of CD34+ bone marrow cells. Blood 84, 1473–1481 (1994).
Hao, Q.L., Shah, A.J., Thiemann, F.T., Smogorzewska, E.M. & Crooks, G.M. A functional comparison of CD34+CD38− Cells in cord blood and bone marrow. Blood 86, 3745–3753 (1995).
Lapidot, T. et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 367, 645–648 (1994).
Sirard, C. et al. Normal and leukemic SCID-repopulating cells (SRCs) coexist in the bone marrow and peripheral blood from CML patients in chronic phase, whereas leukemic SRCs are detected in blast crisis. Blood 87, 1539–1548 (1996).
Nolta, J., Dao, M., Wells, S., Smogorzewska, E. & Kohn, D. Transduction of pluripotent human hematopoietic stem cells demonstrated by clonal analysis after engraftment in immune deficient mice. Proc. Natl Acad. Sci. USA 93, 2414–2419 (1996).
Miller, A.D. et al. Construction and properties of retrovirus packaging cells based on gibbon ape leukemia virus. J. Virol. 65, 2220–2224 (1991).
Lim, B., Apperley, J.F., Orkin, S.H. & Williams, D.A. Long-term expression of human adenosine deaminase in mice transplanted with retrovirus-infected hematopoietic stem cells. Proc. Natl. Acad. Sci. USA 86, 8892–8896 (1989).
Jönsson, J.L., Wu, Q., Nilsson, K. & Phillips, R.A. Use of a promoter-trap retrovirus to identify and isolate genes involved in differentiation of a myeloid progenitor cell line in vitro. Blood 87, 1771–1779 (1996).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Larochelle, A., Vormoor, J., Hanenberg, H. et al. Identification of primitive human hematopoietic cells capable of repopulating NOD/SCID mouse bone marrow: Implications for gene therapy. Nat Med 2, 1329–1337 (1996). https://doi.org/10.1038/nm1296-1329
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm1296-1329
This article is cited by
-
Differentiation therapy for myeloid malignancies: beyond cytotoxicity
Blood Cancer Journal (2021)
-
Patient-derived xenografts as compatible models for precision oncology
Laboratory Animal Research (2020)
-
Expression of mouse CD47 on human cancer cells profoundly increases tumor metastasis in murine models
BMC Cancer (2015)
-
Mutant DNA methylation regulators endow hematopoietic stem cells with the preleukemic stem cell property, a requisite of leukemia initiation and relapse
Frontiers of Medicine (2015)
-
High-titer foamy virus vector transduction and integration sites of human CD34+ cell–derived SCID-repopulating cells
Molecular Therapy - Methods & Clinical Development (2014)