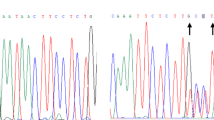
Abstract
By screening members of Finnish families displaying hereditary nonpolyposis colorectal cancer (HNPCC) for predisposing germline mutations in MSH2 and MLH1, we show that two mutations in MLH1 together account for 63% (19/30) of kindreds meeting international diagnostic criteria. Mutation 1, originally detected as a 165-base pair deletion in MLH1 cDNA comprising exon 16, was shown to consist of a 3.5-kilobase genomic deletion most likely resulting from Alu-mediated recombination. Mutation 2 destroys the splice acceptor site of exon 6. A simple diagnostic test based on polymerase chain reaction was designed for both mutations. Our results show that these two ancestral founding mutations account for a majority of Finnish HNPCC kindreds and represent the first report of Alu-mediated recombination causing a prevalent, dominantly inherited predisposition to cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Leach, F.S. et al. Mutations of a MutS homolog in hereditary nonpolyposis colorectal cancer. Cell 75, 1215–1225 (1993).
Bronner, C.E. et al. Mutation in the DNA mismatch repair gene homologue hMLHl is associated with hereditary non-polyposis colon cancer. Nature 368, 258–261 (1994).
Papadopoulos, N. et al. Mutation of a mutL homolog in hereditary colon cancer. Science 263, 1625–1629 (1994).
Nicolaides, N.C. et al. Mutations of two PMS homologues in hereditary nonpolyposis colon cancer. Nature 371, 75–80 (1994).
Aaltonen, L.A. et al. Clues to the pathogenesis of familial colorectal cancer. Science 260, 812–816 (1993).
Nyström-Lahti, M. et al. Mismatch repair genes on chromosomes 2p and 3p accou nt for a major share of hereditary nonpolyposis colorectal cancer families evaluable by linkage. Am. J. hum. Genet. 55, 659–665 (1994).
Liu, B. et al. hMSH2 mutations in hereditary nonpolyposis colorectal cancer kindreds. Cancer Res. 54, 4590–4594 (1994).
Vasen, H.F.A., Mecklin, J.-P., Meera Khan, P. & Lynch, H.T. The international collaborative group on hereditary non-polyposis colorectal cancer (ICG-HNPCC). Dis. Colon Rectum 34, 424–425 (1991).
Nyström-Lahti, M. et al. Close linkage to chromosome 3p and conservation of ancestral founding haplotype in hereditary nonpolyposis colorectal cancer families. Proc. natn. Acad. Sci. U.S.A. 91, 6054–6058 (1994).
Rüdiger, N.S., Gregersen, N. & Kielland-Brandt, M.C. One short well conserved region of Alu-sequences is involved in human gene rearrangements and has homology with prokaryotic chi. Nucleic Acids Res. 23, 256–260 (1995).
Tycko, B., Smith, S.D. & Sklar, J. Chromosomal translocations joining LCK and TCRB loci in human T cell leukemia. J. exp. Med. 174, 867–873 (1991).
Chissoe, S.L. et al. Sequence and analysis of the human ABL gene, the BCR gene, and regions involved in the Philadelphia chromosomal translocation. Genomics 27, 67–82 (1995).
Hemminki, A. et al. Loss of the wild type MLH1 gene is a feature of hereditary nonpolyposis colorectal cancer. Nature Genet. 8, 405–410 (1994).
de la Chapelle, A. Disease gene mapping in isolated human populations: the example of Finland. J. med. Genet. 30, 857–865 (1993).
Froggatt, N.J. et al. A frequent hMSH2 mutation in hereditary non-polyposis colon cancer syndrome. Lancet 345, 727 (1995).
Järvinen, H.J., Mecklin, J.-P. & Sistonen, P. Screening reduces colorectal cancer rate in families with hereditary nonpolyposis colorectal cancer. Gastroenterology 108, 1405–1411 (1995).
Chomczynski, P. & Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162, 156–159 (1987).
Murray, V. Improved double-stranded DNA sequencing using the linear polymerase chain reaction. Nucleic Acids Res. 17, 8889 (1989).
Cheng, S., Fockler, C., Barnes, W.M. & Higuchi, R. Effective amplification of long targets from cloned inserts and human genomic DNA. Proc natn. Acad. Sci. U.S.A. 91, 5695–5699 (1994).
Henikoff, S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene 28, 351–359 (1984).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nyström-Lahti, M., Kristo, P., Nicolaides, N. et al. Founding mutations and Alu-mediated recombination in hereditary colon cancer. Nat Med 1, 1203–1206 (1995). https://doi.org/10.1038/nm1195-1203
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1195-1203
This article is cited by
-
Factors associated with decision-making on prophylactic hysterectomy and attitudes towards gynecological surveillance among women with Lynch syndrome (LS): a descriptive study
Familial Cancer (2020)
-
A survey of the clinicopathological and molecular characteristics of patients with suspected Lynch syndrome in Latin America
BMC Cancer (2017)
-
Milestones of Lynch syndrome: 1895–2015
Nature Reviews Cancer (2015)
-
Expanding the genetic basis of copy number variation in familial breast cancer
Hereditary Cancer in Clinical Practice (2014)
-
A unique MSH2 exon 8 deletion accounts for a major portion of all mismatch repair gene mutations in Lynch syndrome families of Sardinian origin
European Journal of Human Genetics (2013)