Abstract
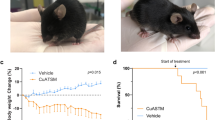
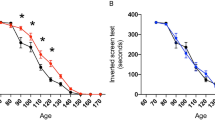
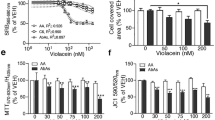
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative condition in which motoneurons of the spinal cord and motor cortex die, resulting in progressive paralysis1,2. This condition has no cure3 and results in eventual death, usually within 1–5 years of diagnosis1,2. Although the specific etiology of ALS is unknown, 20% of familial cases of the disease carry mutations in the gene encoding Cu/Zn superoxide dismutase-1 (SOD1)4. Transgenic mice overexpressing human mutant SOD1 have a phenotype and pathology that are very similar to that seen in human ALS patients5,6. Here we show that treatment with arimoclomol, a coinducer of heat shock proteins (HSPs), significantly delays disease progression in mice expressing a SOD1 mutant in which glycine is substituted with alanine at position 93 (SOD1G93A). Arimoclomol-treated SOD1G93A mice show marked improvement in hind limb muscle function and motoneuron survival in the later stages of the disease, resulting in a 22% increase in lifespan. Pharmacological activation of the heat shock response may therefore be a successful therapeutic approach to treating ALS, and possibly other neurodegenerative diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rowland, L.P. & Shneider, N.A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 344, 1688–1700 (2001).
Shaw, P.J. Motor neurone disease. Br. Med. J. 318, 1118–1121 (1999).
Miller, R.G., Mitchell, J.D., Lyon, M. & Moore, D.H. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst. Rev. CD001447 (2002).
Rosen, D.R. et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362, 59–62 (1993).
Gurney, M.E. et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science 264, 1772–1775 (1994).
Wong, P.C. et al. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron 14, 1105–1116 (1995).
Vigh, L. et al. Bimoclomol: a nontoxic, hydroxylamine derivative with stress protein-inducing activity and cytoprotective effects. Nat. Med. 3, 1150–1154 (1997).
Lindquist, S. The heat-shock response. Annu. Rev. Biochem. 55, 1151–1191 (1986).
Garofalo, O. et al. Ubiquitin and heat shock protein expression in amyotrophic lateral sclerosis. Neuropathol. Appl. Neurobiol. 17, 39–45 (1991).
Vleminckx, V. et al. Upregulation of HSP27 in a transgenic model of ALS. J. Neuropathol. Exp. Neurol. 61, 968–974 (2002).
Shinder, G.A., Lacourse, M.C., Minotti, S. & Durham, H.D. Mutant Cu/Zn- superoxide dismutase proteins have altered solubility and interact with heat shock/stress proteins in models of amyotrophic lateral sclerosis. J. Biol. Chem. 276, 12791–12796 (2001).
Okado-Matsumoto, A. & Fridovich, I. Amyotrophic lateral sclerosis: a proposed mechanism. Proc. Natl. Acad. Sci. USA 99, 9010–9014 (2002).
Kalmar, B. et al. Upregulation of heat shock proteins rescues motoneurones from axotomy-induced cell death in neonatal rats. Exp. Neurol. 176, 87–97 (2002).
Zhu, S. et al. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature 417, 74–78 (2002).
Dick, J., Greensmith, L. & Vrbova, G. Blocking of NMDA receptors during a critical stage of development reduces the effects of nerve injury at birth on muscles and motoneurones. Neuromuscul. Disord. 5, 371–382 (1995).
White, C.M., Greensmith, L. & Vrbova, G. Repeated stimuli for axonal growth causes motoneuron death in adult rats: the effect of botulinum toxin followed by partial denervation. Neuroscience 95, 1101–1109 (2000).
Hargitai, J. et al. Bimoclomol, a heat shock protein co-inducer, acts by the prolonged activation of heat shock factor-1. Biochem. Biophys. Res. Commun. 307, 689–695 (2003).
Batulan, Z. et al. High threshold for induction of the stress response in motor neurons is associated with failure to activate HSF1. J. Neurosci. 23, 5789–5798 (2003).
Sarge, K.D., Murphy, S.P. & Morimoto, R.I. Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress. Mol. Cell Biol. 13, 1392–1407 (1993).
Morrison, A.J., Rush, S.J. & Brown, I.R. Heat shock transcription factors and the HSP70 induction response in brain and kidney of the hyperthermic rat during postnatal development. J. Neurochem. 75, 363–372 (2000).
Acknowledgements
We thank Biorex R&D (Hungary) for the gift of arimoclomal. L.G. is the Graham Watts Senior Research Fellow funded by the Brain Research Trust. D.K. is in receipt of a Brain Research Trust Prize Studentship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
As a consequence of the work presented in this paper, G.B. and L.G. are named on a patent application by Biorex R & D Co., Hungary.
Rights and permissions
About this article
Cite this article
Kieran, D., Kalmar, B., Dick, J. et al. Treatment with arimoclomol, a coinducer of heat shock proteins, delays disease progression in ALS mice. Nat Med 10, 402–405 (2004). https://doi.org/10.1038/nm1021
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1021