Abstract
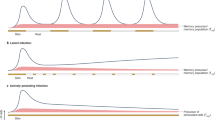
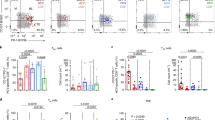
The viruses HIV-1, Epstein–Barr virus (EBV), cytomegalovirus (CMV) and hepatitis C virus (HCV) are characterized by the establishment of lifelong infection in the human host, where their replication is thought to be tightly controlled by virus-specific CD8+ T cells. Here we present detailed studies of the differentiation phenotype of these cells, which can be separated into three distinct subsets based on expression of the costimulatory receptors CD28 and CD27. Whereas CD8+ T cells specific for HIV, EBV and HCV exhibit similar characteristics during primary infection, there are significant enrichments at different stages of cellular differentiation in the chronic phase of persistent infection according to the viral specificity, which suggests that distinct memory T-cell populations are established in different virus infections. These findings challenge the current definitions of memory and effector subsets in humans, and suggest that ascribing effector and memory functions to subsets with different differentiation phenotypes is no longer appropriate.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Koup, R.A. et al. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68, 4650–4655 (1994).
Callan, M.F. et al. Large clonal expansions of CD8+ T cells in acute infectious mononucleosis. Nature Med. 2, 906–911 (1996).
Wills, M.R. et al. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J. Virol. 70, 7569–7579 (1996).
Lechner, F. et al. CD8+ T lymphocyte responses are induced during acute hepatitis C virus infection but are not sustained. Eur. J. Immunol. 30, 2479–2487 (2000).
Appay, V. et al. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 192, 63–75 (2000).
Gillespie, G.M. et al. Functional heterogeneity and high frequencies of cytomegalovirus-specific CD8(+) T lymphocytes in healthy seropositive donors. J. Virol. 74, 8140–8150 (2000).
Hislop, A.D. et al. EBV-specific CD8+ T cell memory: relationships between epitope specificity, cell phenotype, and immediate effector function. J. Immunol. 167, 2019–2029 (2001).
Lechner, F. et al. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 191, 1499–1512 (2000).
Effros, R.B. et al. Shortened telomeres in the expanded CD28−CD8+ cell subset in HIV disease implicate replicative senescence in HIV pathogenesis. AIDS 10, F17–22 (1996).
Posnett, D.N., Edinger, J.W., Manavalan, J.S., Irwin, C. & Marodon, G. Differentiation of human CD8 T cells: implications for in vivo persistence of CD8+ CD28− cytotoxic effector clones. Int. Immunol. 11, 229–241 (1999).
Borthwick, N.J., Lowdell, M., Salmon, M. & Akbar, A.N. Loss of CD28 expression on CD8(+) T cells is induced by IL-2 receptor γ chain signalling cytokines and type I IFN, and increases susceptibility to activation-induced apoptosis. Int. Immunol. 12, 1005–1013 (2000).
Hamann, D. et al. Evidence that human CD8+CD45RA+CD27− cells are induced by antigen and evolve through extensive rounds of division. Int. Immunol. 11, 1027–1033 (1999).
Roos, M.T. et al. Changes in the composition of circulating CD8+ T cell subsets during acute Epstein–Barr and human immunodeficiency virus infections in humans. J. Infect. Dis. 182, 451–458 (2000).
Globerson, A. & Effros, R.B. Ageing of lymphocytes and lymphocytes in the aged. Immunol. Today 21, 515–521 (2000).
De Rosa, S.C., Herzenberg, L.A. & Roederer, M. 11-color, 13-parameter flow cytometry: identification of human naive T cells by phenotype, function, and T-cell receptor diversity. Nature Med. 7, 245–248 (2001).
Ahmed, R. & Gray, D. Immunological memory and protective immunity: Understanding their relation. Science 272, 54–60 (1996).
Doherty, P.C. & Christensen, J.P. Accessing complexity: The dynamics of virus-specific T cell responses. Annu. Rev. Immunol. 18, 561–592 (2000).
Bevan, M.J. & Fink, P.J. The CD8 response on autopilot. Nature Immunol. 2, 381–382 (2001).
Hu, H. et al. CD4(+) T cell effectors can become memory cells with high efficiency and without further division. Nature Immunol. 2, 705–710 (2001).
Hamann, D. et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 186, 1407–1418 (1997).
Sallusto, F., Lenig, D., Forster, R., Lipp, M. & Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999).
Champagne, P. et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature 410, 106–111 (2001).
Strasser, A., O'Connor, L. & Dixit, V.M. Apoptosis signaling. Annu. Rev. Biochem. 69, 217–245 (2000).
Lenschow, D.J., Walunas, T.L. & Bluestone, J.A. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 14, 233–258 (1996).
Hendriks, J. et al. CD27 is required for generation and long-term maintenance of T cell immunity. Nature Immunol. 1, 433–439 (2000).
Ferbas, J. et al. Virus burden in long-term survivors of human immunodeficiency virus (HIV) infection is a determinant of anti-HIV CD8+ lymphocyte activity. J. Infect. Dis. 172, 329–339 (1995).
Medley, Q.G. et al. Characterization of GMP-17, a granule membrane protein that moves to the plasma membrane of natural killer cells following target cell recognition. Proc. Natl. Acad. Sci. USA 93, 685–689 (1996).
Campbell, J.J. et al. 6-C-kine (SLC), a lymphocyte adhesion-triggering chemokine expressed by high endothelium, is an agonist for the MIP-3β receptor CCR7. J. Cell Biol 141, 1053–1059 (1998).
Campbell, J.J. et al. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science 279, 381–384 (1998).
Chen, G. et al. CD8 T cells specific for human immunodeficiency virus, Epstein–Barr virus, and cytomegalovirus lack molecules for homing to lymphoid sites of infection. Blood 98, 156–164 (2001).
Baars, P.A. et al. Cytolytic mechanisms and expression of activation-regulating receptors on effector-type CD8+CD45RA+CD27− human T cells. J. Immunol. 165, 1910–1917 (2000).
Callan, M.F. et al. CD8(+) T-cell selection, function, and death in the primary immune response in vivo. J. Clin. Invest. 106, 1251–1261 (2000).
Mueller, Y.M. et al. Increased CD95/Fas-induced apoptosis of HIV-specific CD8(+) T cells. Immunity 15, 871–882 (2001).
Ridge, J.P., Di Rosa, F. & Matzinger, P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 393, 474–478 (1998).
Kalams, S.A. & Walker, B.D. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J. Exp. Med. 188, 2199–2204 (1998).
Andrews, D.M., Andoniou, C.E., Granucci, F., Ricciardi-Castagnoli, P. & Degli-Esposti, M.A. Infection of dendritic cells by murine cytomegalovirus induces functional paralysis. Nature Immunol. 22, 22 (2001).
Grayson, J.M., Zajac, A.J., Altman, J.D. & Ahmed, R. Cutting edge: Increased expression of Bcl–2 in antigen-specific memory CD8+ T cells. J. Immunol. 164, 3950–3954 (2000).
Homann, D., Teyton, L. & Oldstone, M.B. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nature Med. 7, 913–919 (2001).
Kostense, S. et al. High viral burden in the presence of major HIV-specific CD8(+) T cell expansions: Evidence for impaired CTL effector function. Eur. J. Immunol. 31, 677–686 (2001).
Easterbrook, P.J. Long-term non-progression in HIV infection: Definitions and epidemiological issues. J. Infect. 38, 71–73 (1999).
Bunce, M. et al. Phototyping: Comprehensive DNA typing for HLA-A, B, C, DRB1, DRB3, DRB4, DRB5 & DQB1 by PCR with 144 primer mixes utilizing sequence-specific primers (PCR-SSP). Tissue Antigens 46, 355–367 (1995).
Altman, J.D. et al. Phenotypic analysis of antigen-specific T lymphocytes. Science 274, 94–96 (1996); erratum: 280, 1821 (1998).
Acknowledgements
We thank M. Lucas for help; J. Collier and R. Chapman for blood samples from the Oxford clinic; T. Rostron for HLA typing; B. Walker, G. Lauer, E. Barnes and G. Dusheiko for collaboration; and the staff and patients of the clinics that provided blood samples, particularly the Caldecot Centre at King's College Hospital, the VA Research Center for AIDS and HIV Infection and the UCSD Center for AIDS Research. This work was supported by the Medical Research Council of the UK, the Wellcome Trust, the EU (QLK2-CT-1999-00356), Elizabeth Glaser Pediatric AIDS Foundation and the NIH. S.L.R.-J. is an MRC Senior Fellow and holds an Elizabeth Glaser scientist award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Appay, V., Dunbar, P., Callan, M. et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med 8, 379–385 (2002). https://doi.org/10.1038/nm0402-379
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm0402-379
This article is cited by
-
Correlation of lung immune prognostic index with the efficacy of immune checkpoint inhibitors in Chinese patients with advanced non-small cell lung cancer
Holistic Integrative Oncology (2024)
-
A Novel Biallelic LCK Variant Resulting in Profound T-Cell Immune Deficiency and Review of the Literature
Journal of Clinical Immunology (2024)
-
Deletion of MIF gene from live attenuated LdCen−/− parasites enhances protective CD4+ T cell immunity
Scientific Reports (2023)
-
Regulation of CD8+ T memory and exhaustion by the mTOR signals
Cellular & Molecular Immunology (2023)
-
Estimating the contribution of CD4 T cell subset proliferation and differentiation to HIV persistence
Nature Communications (2023)