Abstract
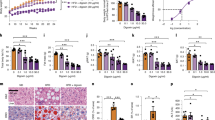
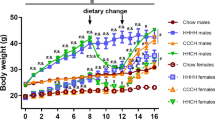
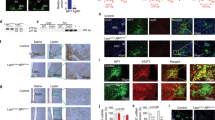
The immune-modulating cytokine interleukin-6 (IL-6) is expressed both in adipose tissue and centrally in hypothalamic nuclei that regulate body composition. We investigated the impact of loss of IL-6 on body composition in mice lacking the gene encoding IL-6 (Il6−/− mice) and found that they developed mature-onset obesity that was partly reversed by IL-6 replacement. The obese Il6−/− mice had disturbed carbohydrate and lipid metabolism, increased leptin levels and decreased responsiveness to leptin treatment. To investigate the possible mechanism and site of action of the anti-obesity effect of IL-6, we injected rats centrally and peripherally with IL-6 at low doses. Intracerebroventricular, but not intraperitoneal IL-6 treatment increased energy expenditure. In conclusion, centrally acting IL-6 exerts anti-obesity effects in rodents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Barsh, G.S., Farooqi, I.S. & O'Rahilly, S. Genetics of body-weight regulation. Nature 404, 644–651 (2000).
Friedman, J.M. & Halaas, J.L. Leptin and the regulation of body weight in mammals. Nature 395, 763–770 (1998).
Robinson, S.W., Dinulescu, D.M. & Cone, R.D. Genetic models of obesity and energy balance in the mouse. Annu. Rev. Genet. 34, 687–745 (2000).
Kopf, M. et al. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 368, 339–342 (1994).
Mohamed-Ali, V. et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J. Clin. Endocrinol. Metab. 82, 4196–4200 (1997).
Fried, S.K., Bunkin, D.A. & Greenberg, A.S. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: Depot difference and regulation by glucocorticoid. J. Clin. Endocrinol. Metab. 83, 847–850 (1998).
Vgontzas, A.N. et al. Sleep apnea and daytime sleepiness and fatigue: Relation to visceral obesity, insulin resistance, and hypercytokinemia. J. Clin. Endocrinol. Metab. 85, 1151–1158 (2000).
Flier, J.S. & Foster, D.W. Eating disorders: Obesity, anorexia nervosa, and bulimia nervosa. in Williams textbook of endocrinology (eds. Williams, R.H., Wilson, J.D., Foster, D.W., Kronenberg, H.M. & Larsen, P.R.) 1061–1097 (Saunders, Philadelphia, 1998).
Schobitz, B., de Kloet, E.R., Sutanto, W. & Holsboer, F. Cellular localization of interleukin 6 mRNA and interleukin 6 receptor mRNA in rat brain. Eur. J. Neurosci. 5, 1426–1435 (1993).
Shizuya, K. et al. The expressions of mRNAs for interleukin-6 (IL-6) and the IL-6 receptor (IL-6R) in the rat hypothalamus and midbrain during restraint stress. Life Sci. 62, 2315–2320 (1998).
Aschner, M. Immune and inflammatory responses in the CNS: Modulation by astrocytes. Toxicol. Lett. 102–103, 283–287 (1998).
Bastard, J.P. et al. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J. Clin. Endocrinol. Metab. 85, 3338–3342 (2000).
Greenberg, A.S. et al. Interleukin 6 reduces lipoprotein lipase activity in adipose tissue of mice in vivo and in 3T3-L1 adipocytes: a possible role for interleukin 6 in cancer cachexia. Cancer Res. 52, 4113–4116 (1992).
Nonogaki, K. et al. Interleukin-6 stimulates hepatic triglyceride secretion in rats. Endocrinology 136, 2143–2149 (1995).
Tsigos, C. et al. Dose-dependent effects of recombinant human interleukin-6 on glucose regulation. J. Clin. Endocrinol. Metab. 82, 4167–4170 (1997).
Chrousos, G.P. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N. Engl. J. Med. 332, 1351–1362 (1995).
Bjorntorp, P. Body fat distribution, insulin resistance, and metabolic diseases. Nutrition 13, 795–803 (1997).
Ershler, W.B. & Keller, E.T. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu. Rev. Med. 51, 245–270 (2000).
Staels, B. et al. Activation of human aortic smooth-muscle cells is inhibited by PPARα but not by PPARγ activators. Nature 393, 790–793 (1998).
McCarty, M.F. Interleukin-6 as a central mediator of cardiovascular risk associated with chronic inflammation, smoking, diabetes, and visceral obesity: down-regulation with essential fatty acids, ethanol and pentoxifylline. Med. Hypotheses 52, 465–477 (1999).
Yudkin, J.S., Kumari, M., Humphries, S.E. & Mohamed-Ali, V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis 148, 209–214 (1999).
Luheshi, G.N., Gardner, J.D., Rushforth, D.A., Loudon, A.S. & Rothwell, N.J. Leptin actions on food intake and body temperature are mediated by IL-1. Proc. Natl. Acad. Sci. USA 96, 7047–7052 (1999).
Rothwell, N.J. et al. Interleukin-6 is a centrally acting endogenous pyrogen in the rat. Can. J. Physiol. Pharmacol. 69, 1465–1469 (1991).
Gloaguen, I. et al. Ciliary neurotrophic factor corrects obesity and diabetes associated with leptin deficiency and resistance. Proc. Natl. Acad. Sci. USA 94, 6456–6461 (1997).
Lambert, P.D. et al. Ciliary neurotrophic factor activates leptin-like pathways and reduces body fat, without cachexia or rebound weight gain, even in leptin- resistant obesity. Proc. Natl. Acad. Sci. USA 98, 4652–4657 (2001).
Bray, G.A. & Tartaglia, L.A. Medicinal strategies in the treatment of obesity. Nature 404, 672–677 (2000).
Sleeman, M.W., Anderson, K.D., Lambert, P.D., Yancopoulos, G.D. & Wiegand, S.J. The ciliary neurotrophic factor and its receptor, CNTFRα. Pharm. Acta Helv. 74, 265–272 (2000).
Espat, N.J. et al. Ciliary neurotrophic factor is catabolic and shares with IL-6 the capacity to induce an acute phase response. Am. J. Physiol. 271, R185–190 (1996).
Bjorbaek, C. et al. Activation of SOCS-3 messenger ribonucleic acid in the hypothalamus by ciliary neurotrophic factor. Endocrinology 140, 2035–2043 (1999).
Xing, Z. et al. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J. Clin. Invest 101, 311–320 (1998).
Pedersen, B.K., Steensberg, A. & Schjerling, P. Muscle-derived interleukin-6: possible biological effects. J. Physiol. 536, 329–337 (2001).
Sjogren, K. et al. Liver-derived IGF-I is of importance for normal carbohydrate and lipid metabolism. Diabetes 50, 1539–1545 (2001).
Ahren, B., Mansson, S., Gingerich, R.L. & Havel, P.J. Regulation of plasma leptin in mice: Influence of age, high-fat diet, and fasting. Am. J. Physiol. 273, R113–120 (1997).
Verdrengh, M. & Tarkowski, A. Role of macrophages in Staphylococcus aureus-induced arthritis and sepsis. Arthritis Rheum. 43, 2276–2282 (2000).
Liao, W., Rudling, M. & Angelin, B. Endotoxin suppresses rat hepatic low-density lipoprotein receptor expression. Biochem. J. 313, 873–878 (1996).
Dickson, S.L., Leng, G., Dyball, R.E. & Smith, R.G. Central actions of peptide and non-peptide growth hormone secretagogues in the rat. Neuroendocrinology 61, 36–43 (1995).
Acknowledgements
We thank M. Kopf for the Il6−/− mice; J. Borén for the measurements of triglycerides; P. Lindström for mouse plasma samples; L. Lönn for valuable advice regarding adipose tissue measurements with CT; L. Kvist, M. Petterson, L. Bengtsson, L. Svensson and I. Berglund-Dahl for technical assistance; and our project student A. Leffler. This study was supported by grants from the European Comission (Framework 5, QLRT-1999-02038), Swedish Research Council (9894, 6834), the Swedish Foundation for International Cooperation in Research and Higher Education (STINT), Bergvall Foundation, Assar Gabrielsson Foundation, Grönberg Foundation, Swedish Medical Society, Gothenburg Medical Society, the Swedish Society for Medical Research, SWEGENE Center for Bio-Imaging (CBI) at Gothenburg University, and the UK Medical Research Council (G9802186).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wallenius, V., Wallenius, K., Ahrén, B. et al. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med 8, 75–79 (2002). https://doi.org/10.1038/nm0102-75
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm0102-75
This article is cited by
-
Glucocorticoids increase adiposity by stimulating Krüppel-like factor 9 expression in macrophages
Nature Communications (2024)
-
Muscle-to-organ cross-talk mediated by interleukin 6 during exercise: a review
Sport Sciences for Health (2024)
-
Effect of aerobic exercise training on the fat fraction of the liver in persons with chronic hepatitis B and hepatic steatosis: Trial protocol for a randomized controlled intervention trial— The FitLiver study
Trials (2023)
-
The Alzheimer’s disease-linked protease BACE1 modulates neuronal IL-6 signaling through shedding of the receptor gp130
Molecular Neurodegeneration (2023)
-
Differential IL18 signaling via IL18 receptor and Na-Cl co-transporter discriminating thermogenesis and glucose metabolism regulation
Nature Communications (2022)