Abstract
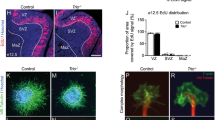
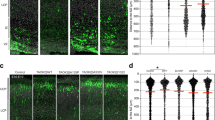
Focal malformations of cortical development (FMCDs) account for the majority of drug-resistant pediatric epilepsy. Postzygotic somatic mutations activating the phosphatidylinositol-4,5-bisphosphate-3-kinase (PI3K)–protein kinase B (AKT)–mammalian target of rapamycin (mTOR) pathway are found in a wide range of brain diseases, including FMCDs. It remains unclear how a mutation in a small fraction of cells disrupts the architecture of the entire hemisphere. Within human FMCD-affected brain, we found that cells showing activation of the PI3K-AKT-mTOR pathway were enriched for the AKT3E17K mutation. Introducing the FMCD-causing mutation into mouse brain resulted in electrographic seizures and impaired hemispheric architecture. Mutation-expressing neural progenitors showed misexpression of reelin, which led to a non–cell autonomous migration defect in neighboring cells, due at least in part to derepression of reelin transcription in a manner dependent on the forkhead box (FOX) transcription factor FOXG1. Treatments aimed at either blocking downstream AKT signaling or inactivating reelin restored migration. These findings suggest a central AKT-FOXG1-reelin signaling pathway in FMCD and support pathway inhibitors as potential treatments or therapies for some forms of focal epilepsy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Lee, J.H. et al. De novo somatic mutations in components of the PI3K-AKT3-mTOR pathway cause hemimegalencephaly. Nat. Genet. 44, 941–945 (2012).
Poduri, A., Evrony, G.D., Cai, X. & Walsh, C.A. Somatic mutation, genomic variation and neurological disease. Science 341, 1237758 (2013).
Aronica, E., Becker, A.J. & Spreafico, R. Malformations of cortical development. Brain Pathol. 22, 380–401 (2012).
Blümcke, I. et al. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc task force of the ILAE Diagnostic Methods Commission. Epilepsia 52, 158–174 (2011).
Taylor, D.C., Falconer, M.A., Bruton, C.J. & Corsellis, J.A. Focal dysplasia of the cerebral cortex in epilepsy. J. Neurol. Neurosurg. Psychiatry 34, 369–387 (1971).
McConnell, M.J. et al. Mosaic copy number variation in human neurons. Science 342, 632–637 (2013).
Rivière, J.B. et al. De novo germline and postzygotic mutations in AKT3, PIK3R2 and PIK3CA cause a spectrum of related megalencephaly syndromes. Nat. Genet. 44, 934–940 (2012).
Poduri, A. et al. Somatic activation of AKT3 causes hemispheric developmental brain malformations. Neuron 74, 41–48 (2012).
Nakashima, M. et al. Somatic mutations in the MTOR gene cause focal cortical dysplasia type IIb. Ann. Neurol. 78, 375–386 (2015).
Lim, J.S. et al. Brain somatic mutations in MTOR cause focal cortical dysplasia type II leading to intractable epilepsy. Nat. Med. 21, 395–400 (2015).
Evrony, G.D. et al. Single-neuron sequencing analysis of L1 retrotransposition and somatic mutation in the human brain. Cell 151, 483–496 (2012).
Cai, X. et al. Single-cell, genome-wide sequencing identifies clonal somatic copy number variation in the human brain. Cell Rep. 8, 1280–1289 (2014).
Conti, V. et al. Focal dysplasia of the cerebral cortex and infantile spasms associated with somatic 1q21.1-q44 duplication including the AKT3 gene. Clin. Genet. 88, 241–247 (2015).
Blümcke, I. et al. Malformations of cortical development and epilepsies: neuropathological findings with emphasis on focal cortical dysplasia. Epileptic Disord. 11, 181–193 (2009).
Hadjivassiliou, G. et al. The application of cortical layer markers in the evaluation of cortical dysplasias in epilepsy. Acta Neuropathol. 120, 517–528 (2010).
Cepeda, C. et al. Epileptogenesis in pediatric cortical dysplasia: the dysmature cerebral developmental hypothesis. Epilepsy Behav. 9, 219–235 (2006).
Chambers, S.M. et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 27, 275–280 (2009).
Tegenge, M.A., Rockel, T.D., Fritsche, E. & Bicker, G. Nitric oxide stimulates human neural progenitor cell migration via cGMP-mediated signal transduction. Cell. Mol. Life Sci. 68, 2089–2099 (2011).
Adams, J.R. et al. Cooperation between Pik3ca and p53 mutations in mouse mammary tumor formation. Cancer Res. 71, 2706–2717 (2011).
D'Arcangelo, G. et al. Reelin is a secreted glycoprotein recognized by the CR-50 monoclonal antibody. J. Neurosci. 17, 23–31 (1997).
Jossin, Y. & Cooper, J.A. Reelin, Rap1 and N-cadherin orient the migration of multipolar neurons in the developing neocortex. Nat. Neurosci. 14, 697–703 (2011).
Hashimoto-Torii, K. et al. Interaction between reelin and Notch signaling regulates neuronal migration in the cerebral cortex. Neuron 60, 273–284 (2008).
Sekine, K. et al. Reelin controls neuronal positioning by promoting cell-matrix adhesion via inside-out activation of integrin-α5β1. Neuron 76, 353–369 (2012).
Franco, S.J., Martinez-Garay, I., Gil-Sanz, C., Harkins-Perry, S.R. & Muller, U. Reelin regulates cadherin function via Dab1/Rap1 to control neuronal migration and lamination in the neocortex. Neuron 69, 482–497 (2011).
Hong, S.E. et al. Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with human RELN mutations. Nat. Genet. 26, 93–96 (2000).
Kubo, K. et al. Ectopic reelin induces neuronal aggregation with a normal birthdate-dependent 'inside-out' alignment in the developing neocortex. J. Neurosci. 30, 10953–10966 (2010).
Sekine, K., Kubo, K.I. & Nakajima, K. How does reelin control neuronal migration and layer formation in the developing mammalian neocortex? Neurosci. Res. 86, 50–58 (2014).
Brunet, A. et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96, 857–868 (1999).
Xuan, S. et al. Winged helix transcription factor BF-1 is essential for the development of the cerebral hemispheres. Neuron 14, 1141–1152 (1995).
Hanashima, C., Li, S.C., Shen, L., Lai, E. & Fishell, G. Foxg1 suppresses early cortical cell fate. Science 303, 56–59 (2004).
Regad, T., Roth, M., Bredenkamp, N., Illing, N. & Papalopulu, N. The neural progenitor–specifying activity of FoxG1 is antagonistically regulated by CKI and FGF. Nat. Cell Biol. 9, 531–540 (2007).
Fauser, S. et al. Long-term seizure outcome in 211 patients with focal cortical dysplasia. Epilepsia 56, 66–76 (2015).
Gutmann, D.H. Tumor suppressor genes as negative growth regulators in development and differentiation. Int. J. Dev. Biol. 39, 895–908 (1995).
Kwon, C.H. et al. Pten regulates neuronal soma size: a mouse model of Lhermitte-Duclos disease. Nat. Genet. 29, 404–411 (2001).
Backman, S.A. et al. Deletion of Pten in mouse brain causes seizures, ataxia and defects in soma size resembling Lhermitte-Duclos disease. Nat. Genet. 29, 396–403 (2001).
Feliciano, D.M., Su, T., Lopez, J., Platel, J.C. & Bordey, A. Single-cell Tsc1 knockout during corticogenesis generates tuber-like lesions and reduces seizure threshold in mice. J. Clin. Invest. 121, 1596–1607 (2011).
Bateup, H.S. et al. Excitatory-inhibitory synaptic imbalance leads to hippocampal hyperexcitability in mouse models of tuberous sclerosis. Neuron 78, 510–522 (2013).
Tavazoie, S.F., Alvarez, V.A., Ridenour, D.A., Kwiatkowski, D.J. & Sabatini, B.L. Regulation of neuronal morphology and function by the tumor suppressors Tsc1 and Tsc2. Nat. Neurosci. 8, 1727–1734 (2005).
Fraser, M.M. et al. Pten loss causes hypertrophy and increased proliferation of astrocytes in vivo. Cancer Res. 64, 7773–7779 (2004).
Zhou, J. & Parada, L.F. PTEN signaling in autism spectrum disorders. Curr. Opin. Neurobiol. 22, 873–879 (2012).
Kwon, C.H. et al. Pten regulates neuronal arborization and social interaction in mice. Neuron 50, 377–388 (2006).
Tsai, P.T. et al. Autistic-like behavior and cerebellar dysfunction in Purkinje cell Tsc1-mutant mice. Nature 488, 647–651 (2012).
Ariani, F. et al. FOXG1 is responsible for the congenital variant of Rett syndrome. Am. J. Hum. Genet. 83, 89–93 (2008).
Persico, A.M. et al. Reelin gene alleles and haplotypes as a factor predisposing to autistic disorder. Mol. Psychiatry 6, 150–159 (2001).
Crunelli, V. & Leresche, N. Childhood absence epilepsy: genes, channels, neurons and networks. Nat. Rev. Neurosci. 3, 371–382 (2002).
Truccolo, W. et al. Single-neuron dynamics in human focal epilepsy. Nat. Neurosci. 14, 635–641 (2011).
Matsuki, T. et al. Reelin and Stk25 have opposing roles in neuronal polarization and dendritic Golgi deployment. Cell 143, 826–836 (2010).
Kupferman, J.V. et al. Reelin signaling specifies the molecular identity of the pyramidal neuron distal dendritic compartment. Cell 158, 1335–1347 (2014).
Pujadas, L. et al. Reelin regulates postnatal neurogenesis and enhances spine hypertrophy and long-term potentiation. J. Neurosci. 30, 4636–4649 (2010).
Brennan, C.W. et al. The somatic genomic landscape of glioblastoma. Cell 155, 462–477 (2013).
Chow, L.M. et al. Cooperativity within and among Pten, p53 and Rb pathways induces high-grade astrocytoma in adult brain. Cancer Cell 19, 305–316 (2011).
Friedmann-Morvinski, D. et al. Dedifferentiation of neurons and astrocytes by oncogenes can induce gliomas in mice. Science 338, 1080–1084 (2012).
Kurek, K.C. et al. Somatic mosaic activating mutations in PIK3CA cause CLOVES syndrome. Am. J. Hum. Genet. 90, 1108–1115 (2012).
Lindhurst, M.J. et al. A mosaic activating mutation in AKT1 associated with the Proteus syndrome. N. Engl. J. Med. 365, 611–619 (2011).
Keppler-Noreuil, K.M. et al. Clinical delineation and natural history of the PIK3CA-related overgrowth spectrum. Am. J. Med. Genet. A. 164, 1713–1733 (2014).
LoTurco, J.J. & Bai, J. The multipolar stage and disruptions in neuronal migration. Trends Neurosci. 29, 407–413 (2006).
National Research Council. Guide for the Care and Use of Laboratory Animals 8th edn. (The National Academies Press, 2011).
Baek, S.T. et al. Off-target effect of doublecortin family shRNA on neuronal migration associated with endogenous microRNA dysregulation. Neuron 82, 1255–1262 (2014).
Sarnat, H.B. Clinical neuropathology practice guide 5-2013: markers of neuronal maturation. Clin. Neuropathol. 32, 340–369 (2013).
Zhang, Y. et al. An RNA-sequencing transcriptome and splicing database of glia, neurons and vascular cells of the cerebral cortex. J. Neurosci. 34, 11929–11947 (2014).
Trapnell, C. et al. Transcript assembly and quantification by RNA-seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28, 511–515 (2010).
Acknowledgements
We thank N. Cai, M. Huynh, T. Chirwa, K. Um, J. Silhavy and U. Yang for technical expertise. This work was supported by the US National Institutes of Health (grant no. R01NS083823; J.G.G. and G.W.M.), the Simons Foundation for Autism Research (grant no. 275275; J.G.G.), the Howard Hughes Medical Institute (J.G.G.), the 2014 National Alliance for Research on Schizophrenia and Depression (NARSAD) Young Investigator Grant from the Brain & Behavior Research Foundation (grant no. 22892; S.T.B.), a Human Frontier Science Program Long-Term Fellowship (S.-K.K.), an A.P. Giannini Foundation Fellowship (A.E.S.) and an NIH NICHD K99/R00 Pathway to Independence Award (grant no. K99HD082337; A.E.S.). G.W.M. was supported by the Dr. Alfonsina Q. Davies Endowed Chair in honor of Paul Crandall MD for Epilepsy Research. We thank the UCSD Neuroscience Microscopy Core P30 NS047101 for imaging support, K. Jepsen from the UCSD Institute for Genomic Medicine Core Facility, the UCSD Human Embryonic Stem Cell Core Facility, A. Roberts from the Scripps Research Institute Animal Core Facility, I. Verma (Salk Institute) for the pBOB-Switch vector, A. Acharya (University of Texas Southwestern Medical Center) for the Cre-expressing adenovirus, G. Fishell (New York University) for the Foxg1 plasmid, and P. Mischel, I. Martin-Valencia, T. Curran and T. Park for discussions.
Author information
Authors and Affiliations
Contributions
S.T.B. and J.G.G. designed experiments, analyzed data and wrote the manuscript. B.C. performed bioinformatics analysis of RNA-seq results. S.T.B. performed experiments. E.-J.Y. performed FOXG1 chromatin immunoprecipitation. S.-K.K. helped with in utero electroporation. A.G.-G., A.E.S., S.K., H.-C.K., S.S. and G.W.M. contributed key reagents and advice. J.G.G. conceived and supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 (PDF 5601 kb)
Rights and permissions
About this article
Cite this article
Baek, S., Copeland, B., Yun, EJ. et al. An AKT3-FOXG1-reelin network underlies defective migration in human focal malformations of cortical development. Nat Med 21, 1445–1454 (2015). https://doi.org/10.1038/nm.3982
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3982