Abstract
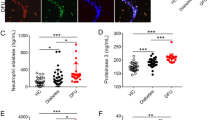
Wound healing is impaired in diabetes, resulting in significant morbidity and mortality. Neutrophils are the main leukocytes involved in the early phase of healing. As part of their anti-microbial defense, neutrophils form extracellular traps (NETs) by releasing decondensed chromatin lined with cytotoxic proteins1. NETs, however, can also induce tissue damage. Here we show that neutrophils isolated from type 1 and type 2 diabetic humans and mice were primed to produce NETs (a process termed NETosis). Expression of peptidylarginine deiminase 4 (PAD4, encoded by Padi4 in mice), an enzyme important in chromatin decondensation, was elevated in neutrophils from individuals with diabetes. When subjected to excisional skin wounds, wild-type (WT) mice produced large quantities of NETs in wounds, but this was not observed in Padi4−/− mice. In diabetic mice, higher levels of citrullinated histone H3 (H3Cit, a NET marker) were found in their wounds than in normoglycemic mice and healing was delayed. Wound healing was accelerated in Padi4−/− mice as compared to WT mice, and it was not compromised by diabetes. DNase 1, which disrupts NETs, accelerated wound healing in diabetic and normoglycemic WT mice. Thus, NETs impair wound healing, particularly in diabetes, in which neutrophils are more susceptible to NETosis. Inhibiting NETosis or cleaving NETs may improve wound healing and reduce NET-driven chronic inflammation in diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Brinkmann, V. et al. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 (2004).
Wang, Y. et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J. Cell Biol. 184, 205–213 (2009).
Wang, Y. et al. Human PAD4 regulates histone arginine methylation levels via demethylimination. Science 306, 279–283 (2004).
Yipp, B.G. & Kubes, P. NETosis: how vital is it? Blood 122, 2784–2794 (2013).
Martinod, K. & Wagner, D.D. Thrombosis: tangled up in NETs. Blood 123, 2768–2776 (2014).
Karima, M. et al. Enhanced superoxide release and elevated protein kinase C activity in neutrophils from diabetic patients: association with periodontitis. J. Leukoc. Biol. 78, 862–870 (2005).
Hanses, F., Park, S., Rich, J. & Lee, J.C. Reduced neutrophil apoptosis in diabetic mice during staphylococcal infection leads to prolonged TNFα production and reduced neutrophil clearance. PLoS ONE 6, e23633 (2011).
Thomas, G.M. et al. Extracellular DNA traps are associated with the pathogenesis of TRALI in humans and mice. Blood 119, 6335–6343 (2012).
Alexandraki, K.I. et al. Cytokine secretion in long-standing diabetes mellitus type 1 and 2: associations with low-grade systemic inflammation. J. Clin. Immunol. 28, 314–321 (2008).
Luo, Y. et al. Inhibitors and inactivators of protein arginine deiminase 4: functional and structural characterization. Biochemistry 45, 11727–11736 (2006).
Li, P. et al. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 207, 1853–1862 (2010).
Leshner, M. et al. PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Front Immunol 3, 307 (2012).
Alexiewicz, J.M., Kumar, D., Smogorzewski, M., Klin, M. & Massry, S.G. Polymorphonuclear leukocytes in non-insulin-dependent diabetes mellitus: abnormalities in metabolism and function. Ann. Intern. Med. 123, 919–924 (1995).
Gupta, A.K., Giaglis, S., Hasler, P. & Hahn, S. Efficient neutrophil extracellular trap induction requires mobilization of both intracellular and extracellular calcium pools and is modulated by cyclosporine A. PLoS ONE 9, e97088 (2014).
Rodríguez-Espinosa, O., Rojas-Espinosa, O., Moreno-Altamirano, M.M., Lopez-Villegas, E.O. & Sanchez-Garcia, F.J. Metabolic requirements for neutrophil extracellular traps (NETs) formation. Immunology 145, 213–224 (2015).
Menegazzo, L. et al. NETosis is induced by high glucose and associated with type 2 diabetes. Acta Diabetol. (in the press 2014).
Joshi, M.B. et al. High glucose modulates IL-6 mediated immune homeostasis through impeding neutrophil extracellular trap formation. FEBS Lett. 587, 2241–2246 (2013).
Riyapa, D. et al. Neutrophil extracellular traps exhibit antibacterial activity against Burkholderia pseudomallei and are influenced by bacterial and host factors. Infect. Immun. 80, 3921–3929 (2012).
Fuchs, T.A. et al. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 176, 231–241 (2007).
Rebecchi, I.M., Ferreira Novo, N., Julian, Y. & Campa, A. Oxidative metabolism and release of myeloperoxidase from polymorphonuclear leukocytes obtained from blood sedimentation in a Ficoll-Hypaque gradient. Cell Biochem. Funct. 18, 127–132 (2000).
Brinkmann, V., Laube, B., Abu Abed, U., Goosmann, C. & Zychlinsky, A. Neutrophil extracellular traps: how to generate and visualize them. J. Vis. Exp., 1724 (2010).
Dovi, J.V., He, L.K. & DiPietro, L.A. Accelerated wound closure in neutrophil-depleted mice. J. Leukoc. Biol. 73, 448–455 (2003).
Saffarzadeh, M. et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS ONE 7, e32366 (2012).
Subramaniam, M. et al. Role of endothelial selectins in wound repair. Am. J. Pathol. 150, 1701–1709 (1997).
Nachat, R. et al. Peptidylarginine deiminase isoforms 1–3 are expressed in the epidermis and involved in the deimination of K1 and filaggrin. J. Invest. Dermatol. 124, 384–393 (2005).
Frenette, P.S., Mayadas, T.N., Rayburn, H., Hynes, R.O. & Wagner, D.D. Susceptibility to infection and altered hematopoiesis in mice deficient in both P- and E-selectins. Cell 84, 563–574 (1996).
Martinod, K. et al. PAD4-deficiency does not affect bacteremia in polymicrobial sepsis and ameliorates endotoxemic shock. Blood 125, 1948–1956 (2015).
Coudane, F. et al. Deimination and expression of peptidylarginine deiminases during cutaneous wound healing in mice. Eur. J. Dermatol. 21, 376–384 (2011).
Herrick, S. et al. Up-regulation of elastase in acute wounds of healthy aged humans and chronic venous leg ulcers are associated with matrix degradation. Lab. Invest. 77, 281–288 (1997).
Grice, E.A. et al. Longitudinal shift in diabetic wound microbiota correlates with prolonged skin defense response. Proc. Natl. Acad. Sci. USA 107, 14799–14804 (2010).
Berends, E.T. et al. Nuclease expression by Staphylococcus aureus facilitates escape from neutrophil extracellular traps. J. Innate Immun. 2, 576–586 (2010).
Farrera, C. & Fadeel, B. Macrophage clearance of neutrophil extracellular traps is a silent process. J. Immunol. 191, 2647–2656 (2013).
Bodaño, A. et al. Study of DNASE I gene polymorphisms in systemic lupus erythematosus susceptibility. Ann. Rheum. Dis. 66, 560–561 (2007).
Kumamoto, T. et al. Association of Gln222Arg polymorphism in the deoxyribonuclease I (DNase I) gene with myocardial infarction in Japanese patients. Eur. Heart J. 27, 2081–2087 (2006).
Hakkim, A. et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl. Acad. Sci. USA 107, 9813–9818 (2010).
Lewis, H.D. et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat. Chem. Biol. 11, 189–191 (2015).
Laakso, M. & Kuusisto, J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat. Rev. Endocrinol. 10, 293–302 (2014).
Morel, O., Jesel, L., Abbas, M. & Morel, N. Prothrombotic changes in diabetes mellitus. Semin. Thromb. Hemost. 39, 477–488 (2013).
Demers, M. et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc. Natl. Acad. Sci. USA 109, 13076–13081 (2012).
Brill, A. et al. Neutrophil extracellular traps promote deep vein thrombosis in mice. J. Thromb. Haemost. 10, 136–144 (2012).
Acknowledgements
We thank H. Ferris for advice on diabetes protocols; L. DeVita for selection of diabetic patients; P. Forbes (The Harvard Clinical and Translational Science Center, US National Institutes of Health (NIH) Award UL1 TR001102) for statistical advice; J.E. Cabral and S. Cifuni for valuable technical support; and L. Cowan for manuscript preparation assistance. This study was supported by the American Diabetes Association (Innovation Award 7-13-IN-44 to D.D.W.), the National Heart, Lung, and Blood Institute of the NIH (R01HL102101 to D.D.W.), the National Cancer Institute (R01CA136856 to Y.W.), the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK031036 to C.R.K.) and a GlaxoSmithKline/Immune Disease Institute Alliance Fellowship (S.L.W.).
Author information
Authors and Affiliations
Contributions
S.L.W. designed the study, performed the majority of the experiments, analyzed the data and wrote the manuscript; M.D. and K.M. performed experiments and analyzed data; M.G. provided expert technical assistance; Y.W. provided Padi4−/− mice and critical discussion of the work; A.B.G. provided clinical advice and selected diabetic patients for in vitro NETosis assays; C.R.K. provided helpful suggestions on experimental design and critical reading of the manuscript; D.D.W. designed the study, supervised the project and co-wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Supplementary Table 1 (PDF 18613 kb)
Rights and permissions
About this article
Cite this article
Wong, S., Demers, M., Martinod, K. et al. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med 21, 815–819 (2015). https://doi.org/10.1038/nm.3887
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3887
This article is cited by
-
Prognostic implications of systemic immune-inflammation index in myocardial infarction patients with and without diabetes: insights from the NOAFCAMI-SH registry
Cardiovascular Diabetology (2024)
-
A novel diabetic foot ulcer diagnostic model: identification and analysis of genes related to glutamine metabolism and immune infiltration
BMC Genomics (2024)
-
CGRP sensory neurons promote tissue healing via neutrophils and macrophages
Nature (2024)
-
NLRP3 inflammasome activation and NETosis positively regulate each other and exacerbate proinflammatory responses: implications of NETosis inhibition for acne skin inflammation treatment
Cellular & Molecular Immunology (2024)
-
Quantitative evaluation of citrullinated fibrinogen for detection of neutrophil extracellular traps
Immunologic Research (2024)