Abstract
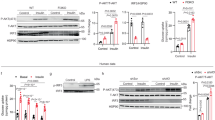
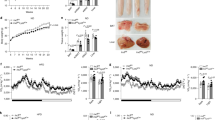
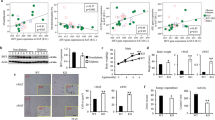
Accumulation of visceral adipose tissue correlates with elevated inflammation and increased risk of metabolic diseases. However, little is known about the molecular mechanisms that control its pathological expansion. Transcription factor interferon regulatory factor 5 (IRF5) has been implicated in polarizing macrophages towards an inflammatory phenotype. Here we demonstrate that mice lacking Irf5, when placed on a high-fat diet, show no difference in the growth of their epididymal white adipose tissue (epiWAT) but they show expansion of their subcutaneous white adipose tissue, as compared to wild-type (WT) mice on the same diet. EpiWAT from Irf5-deficient mice is marked by accumulation of alternatively activated macrophages, higher collagen deposition that restricts adipocyte size, and enhanced insulin sensitivity compared to epiWAT from WT mice. In obese individuals, IRF5 expression is negatively associated with insulin sensitivity and collagen deposition in visceral adipose tissue. Genome-wide analysis of gene expression in adipose tissue macrophages highlights the transforming growth factor β1 (TGFB1) gene itself as a direct target of IRF5-mediated inhibition. This study uncovers a new function for IRF5 in controlling the relative mass of different adipose tissue depots and thus insulin sensitivity in obesity, and it suggests that inhibition of IRF5 may promote a healthy metabolic state during this condition.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Tchernof, A. & Despres, J.P. Pathophysiology of human visceral obesity: an update. Physiol. Rev. 93, 359–404 (2013).
Klöting, N. et al. Insulin-sensitive obesity. Am. J. Physiol. Endocrinol. Metab. 299, E506–E515 (2010).
Abate, N., Garg, A., Peshock, R.M., Stray-Gundersen, J. & Grundy, S.M. Relationships of generalized and regional adiposity to insulin sensitivity in men. J. Clin. Invest. 96, 88–98 (1995).
Lawrence, T. & Natoli, G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat. Rev. Immunol. 11, 750–761 (2011).
Odegaard, J.I. & Chawla, A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science 339, 172–177 (2013).
Liao, X. et al. Kruppel-like factor 4 regulates macrophage polarization. J. Clin. Invest. 121, 2736–2749 (2011).
Odegaard, J.I. et al. Macrophage-specific PPAR-γ controls alternative activation and improves insulin resistance. Nature 447, 1116–1120 (2007).
Graham, R.R. et al. A common haplotype of interferon regulatory factor 5 (IRF5) regulates splicing and expression and is associated with increased risk of systemic lupus erythematosus. Nat. Genet. 38, 550–555 (2006).
Krausgruber, T. et al. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat. Immunol. 12, 231–238 (2011).
Bertola, A. et al. Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes 61, 2238–2247 (2012).
Dalmas, E. et al. T cell-derived IL-22 amplifies IL-1β-driven inflammation in human adipose tissue: relevance to obesity and type 2 diabetes. Diabetes 63, 1966–1977 (2014).
Fabbrini, E. et al. Association between specific adipose tissue CD4+ T-cell populations and insulin resistance in obese individuals. Gastroenterology 145, 366–374 e1–3 (2013).
Bourlier, V. et al. Remodeling phenotype of human subcutaneous adipose tissue macrophages. Circulation 117, 806–815 (2008).
Xu, X. et al. Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab. 18, 816–830 (2013).
Kim, J.Y. et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Invest. 117, 2621–2637 (2007).
Wander, P.L. et al. Change in visceral adiposity independently predicts a greater risk of developing type 2 diabetes over 10 years in Japanese Americans. Diabetes Care 36, 289–293 (2013).
Arner, P., Arner, E., Hammarstedt, A. & Smith, U. Genetic predisposition for type 2 diabetes, but not for overweight/obesity, is associated with a restricted adipogenesis. PLoS ONE 6, e18284 (2011).
Hoffstedt, J. et al. Regional impact of adipose tissue morphology on the metabolic profile in morbid obesity. Diabetologia 53, 2496–2503 (2010).
Sun, K., Tordjman, J., Clement, K. & Scherer, P.E. Fibrosis and adipose tissue dysfunction. Cell Metab. 18, 470–477 (2013).
Henegar, C. et al. Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity. Genome Biol. 9, R14 (2008).
Chun, T.H. et al. A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell 125, 577–591 (2006).
Vila, I.K. et al. Immune cell toll-like receptor 4 mediates the development of obesity- and endotoxemia-associated adipose tissue fibrosis. Cell Rep. 7, 1116–1129 (2014).
Khan, T. et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol. Cell. Biol. 29, 1575–1591 (2009).
Lee, J.T. et al. Macrophage metalloelastase (MMP12) regulates adipose tissue expansion, insulin sensitivity, and expression of inducible nitric oxide synthase. Endocrinology 155, 3409–3420 (2014).
Divoux, A. et al. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes 59, 2817–2825 (2010).
Arner, E. et al. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes 59, 105–109 (2010).
Tordjman, J. et al. Structural and inflammatory heterogeneity in subcutaneous adipose tissue: relation with liver histopathology in morbid obesity. J. Hepatol. 56, 1152–1158 (2012).
Halberg, N. et al. Hypoxia-inducible factor 1α induces fibrosis and insulin resistance in white adipose tissue. Mol. Cell. Biol. 29, 4467–4483 (2009).
Wernstedt Asterholm, I. et al. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 20, 103–118 (2014).
Wynn, T.A. & Barron, L. Macrophages: master regulators of inflammation and fibrosis. Semin. Liver Dis. 30, 245–257 (2010).
Song, E. et al. Influence of alternatively and classically activated macrophages on fibrogenic activities of human fibroblasts. Cell. Immunol. 204, 19–28 (2000).
Keophiphath, M. et al. Macrophage-secreted factors promote a profibrotic phenotype in human preadipocytes. Mol. Endocrinol. 23, 11–24 (2009).
Lazear, H.M. et al. IRF-3, IRF-5, and IRF-7 coordinately regulate the type I IFN response in myeloid dendritic cells downstream of MAVS signaling. PLoS Pathog. 9, e1003118 (2013).
Takaoka, A. et al. Integral role of IRF-5 in the gene induction programme activated by toll-like receptors. Nature 434, 243–249 (2005).
Spencer, M. et al. Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance. J. Clin. Endocrinol. Metab. 96, E1990–E1998 (2011).
Seki, E. et al. TLR4 enhances TGF-β signaling and hepatic fibrosis. Nat. Med. 13, 1324–1332 (2007).
Wu, D. et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 332, 243–247 (2011).
Petruschke, T., Rohrig, K. & Hauner, H. Transforming growth factor beta (TGF-beta) inhibits the differentiation of human adipocyte precursor cells in primary culture. Int. J. Obes. Relat. Metab. Disord. 18, 532–536 (1994).
Ignotz, R.A. & Massague, J. Type beta transforming growth factor controls the adipogenic differentiation of 3T3 fibroblasts. Proc. Natl. Acad. Sci. USA 82, 8530–8534 (1985).
Klimčáková, E. et al. Worsening of obesity and metabolic status yields similar molecular adaptations in human subcutaneous and visceral adipose tissue: decreased metabolism and increased immune response. J. Clin. Endocrinol. Metab. 96, E73–E82 (2011).
Klimcáková, E. et al. Macrophage gene expression is related to obesity and the metabolic syndrome in human subcutaneous fat as well as in visceral fat. Diabetologia 54, 876–887 (2011).
Krausgruber, T. et al. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat. Immunol. 12, 231–238 (2011).
Joly-Amado, A. et al. Hypothalamic AgRP-neurons control peripheral substrate utilization and nutrient partitioning. EMBO J. 31, 4276–4288 (2012).
Berglund, E.D. et al. Glucose metabolism in vivo in four commonly used inbred mouse strains. Diabetes 57, 1790–1799 (2008).
Hajduch, E., Darakhshan, F. & Hundal, H.S. Fructose uptake in rat adipocytes: GLUT5 expression and the effects of streptozotocin-induced diabetes. Diabetologia 41, 821–828 (1998).
Kammoun, H.L. et al. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J. Clin. Invest. 119, 1201–1215 (2009).
Divoux, A. et al. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes 59, 2817–2825 (2010).
Rouault, C. et al. Roles of chemokine ligand-2 (CXCL2) and neutrophils in influencing endothelial cell function and inflammation of human adipose tissue. Endocrinology 154, 1069–1079 (2013).
Prieur, X. et al. Differential lipid partitioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and M2/M1 polarization in obese mice. Diabetes 60, 797–809 (2011).
Toubal, A. et al. SMRT-GPS2 corepressor pathway dysregulation coincides with obesity-linked adipocyte inflammation. J. Clin. Invest. 123, 362–379 (2013).
Storey, J.D. in International Encyclopedia of Statistical Science 1st edn, (ed. Lovric, M.) False Discovery Rate (Springer, 2011).
Prifti, E., Zucker, J.D., Clement, K. & Henegar, C. FunNet: an integrative tool for exploring transcriptional interactions. Bioinformatics 24, 2636–2638 (2008).
Arch, J.R., Hislop, D., Wang, S.J. & Speakman, J.R. Some mathematical and technical issues in the measurement and interpretation of open-circuit indirect calorimetry in small animals. Int. J. Obes. (Lond) 30, 1322–1331 (2006).
Even, P.C., Mokhtarian, A. & Pele, A. Practical aspects of indirect calorimetry in laboratory animals. Neurosci. Biobehav. Rev. 18, 435–447 (1994).
Weir, J.B. New methods for calculating metabolic rate with special reference to protein metabolism. 1949. Nutrition 6, 213–221 (1990).
Acknowledgements
We are grateful to C. Enond and H. Foher-Ting. We thank Assistance Publique des Hôpitaux de Paris, Programs of Clinical Investigation (Contrat de Recherche Clinique Fibrota AOO759-32 PI to J.A.-W. and Programme Hospitalier de Recherche Clinique 0702 PI to K.C.), the French National Agency of Research (CONRAD to N. Venteclef, ADIPOFIB to K.C. and OBELIP to D.L.), a fellowship from Region Ile de France (to F.A., N. Venteclef and K.C.), Midi-Pyrénées (to D.L.), and the French Foundation for Medical Research (Equipe FRM DEQ20140329504 to F.F. and N. Venteclef and Equipe FRM DEQ20120323701 to K.C.). This work has benefited from a French Government grant managed by the National Agency for Research ('Investments for the Future' program, reference no. ANR-10-IAHU (to K.C. and F.F.) and from the Kennedy Institute Trustees' Research Fund (to K.B., H.L.E. and I.A.U.). Metabolic analyses in vivo were performed on the Functional & Physiological Exploration Platform (FPE) technical platform, unit 'Biologie Fonctionnelle et Adaptative,' (Univ. Paris Diderot, Sorbonne Paris Cité, Paris, France), and on the ICAN PRECLINIC platform (Institute of Cardiometabolism and Nutrition, Pitie-Salpêtrière Hospital, Paris, France). D.L. is a member of Institut Universitaire de France. A. Toubal is a recipient of a doctoral fellowship from the Ministère de la Recherche et de l'Enseignement Supérieur.
Author information
Authors and Affiliations
Contributions
E.D., I.A.U. and N. Venteclef conceived the study and wrote the manuscript. E.D. performed part of the in vivo studies (human and mouse) and analyzed data. A. Toubal and F.A. performed the in vivo studies and data analyses, and assisted in the preparation of the manuscript. K.B., H.L.E., M.P., I.H., Y.L. and P.A. assisted with the mouse studies. S.A. and K.L. assisted with flow cytometry analyses. A.L., R.G.P.D. and C.C.-G. performed the metabolic analyses in vivo. E.M., N. Viguerie, C.P., V.S., A. Torcivia, J.A.-W., D.L. and K.C. contributed to the human data collection, data analyses and interpretation. O.A. and K.C. performed statistical analyses in population 1. F.F., S.L., J.A.-W., D.L. and K.C. interpreted and assisted in the writing of the manuscript. I.A.U. and N. Venteclef designed, analyzed and interpreted the studies.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Table 1 (PDF 1582 kb)
Rights and permissions
About this article
Cite this article
Dalmas, E., Toubal, A., Alzaid, F. et al. Irf5 deficiency in macrophages promotes beneficial adipose tissue expansion and insulin sensitivity during obesity. Nat Med 21, 610–618 (2015). https://doi.org/10.1038/nm.3829
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3829
This article is cited by
-
Genetic variants of interferon-response factor 5 are associated with the incidence of chronic kidney disease: the D.E.S.I.R. study
Genes & Immunity (2023)
-
Macrophage function in adipose tissue homeostasis and metabolic inflammation
Nature Immunology (2023)
-
Gut bacteria-derived 3-phenylpropionylglycine mitigates adipocyte differentiation of 3T3-L1 cells by inhibiting adiponectin-PPAR pathway
Genes & Genomics (2023)
-
Early macrophage response to obesity encompasses Interferon Regulatory Factor 5 regulated mitochondrial architecture remodelling
Nature Communications (2022)
-
High carbohydrate intakes may predict more inflammatory status than high fat intakes in pre-menopause women with overweight or obesity: a cross-sectional study
BMC Research Notes (2021)