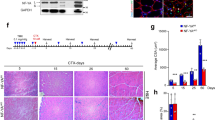
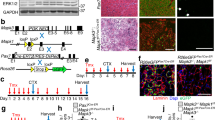
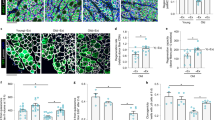
Abstract
Diminished regenerative capacity of skeletal muscle occurs during adulthood. We identified a reduction in the intrinsic capacity of mouse adult satellite cells to contribute to muscle regeneration and repopulation of the niche. Gene expression analysis identified higher expression of JAK-STAT signaling targets in 18-month-old relative to 3-week-old mice. Knockdown of Jak2 or Stat3 significantly stimulated symmetric satellite stem cell divisions on cultured myofibers. Genetic knockdown of Jak2 or Stat3 expression in prospectively isolated satellite cells markedly enhanced their ability to repopulate the satellite cell niche after transplantation into regenerating tibialis anterior muscle. Pharmacological inhibition of Jak2 and Stat3 activity similarly stimulated symmetric expansion of satellite cells in vitro and their engraftment in vivo. Intramuscular injection of these drugs resulted in a marked enhancement of muscle repair and force generation after cardiotoxin injury. Together these results reveal age-related intrinsic properties that functionally distinguish satellite cells and suggest a promising therapeutic avenue for the treatment of muscle-wasting diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
Change history
22 September 2014
In the version of this article initially published online, the third sentence of the Abstract read “Gene expression analysis identified higher expression of JAK-STAT signaling targets in 3-week-old relative to 18-month-old mice,” when it should have read “Gene expression analysis identified higher expression of JAK-STAT signaling targets in 18-month-old relative to 3-week-old mice.” The error has been corrected for the print, PDF and HTML versions of this article.
References
Mauro, A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 9, 493–495 (1961).
Schultz, E., Gibson, M.C. & Champion, T. Satellite cells are mitotically quiescent in mature mouse muscle: an EM and radioautographic study. J. Exp. Zool. 206, 451–456 (1978).
Montarras, D. et al. Direct isolation of satellite cells for skeletal muscle regeneration. Science 309, 2064–2067 (2005).
Collins, C.A. et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 122, 289–301 (2005).
Kuang, S., Kuroda, K., Le Grand, F. & Rudnicki, M.A. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell 129, 999–1010 (2007).
Zammit, P.S. et al. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J. Cell Biol. 166, 347–357 (2004).
Carlson, M.E. & Conboy, I.M. Loss of stem cell regenerative capacity within aged niches. Aging Cell 6, 371–382 (2007).
Augustin, H. & Partridge, L. Invertebrate models of age-related muscle degeneration. Biochim. Biophys. Acta 1790, 1084–1094 (2009).
Grounds, M.D. Age-associated changes in the response of skeletal muscle cells to exercise and regeneration. Ann. NY Acad. Sci. 854, 78–91 (1998).
Allbrook, D.B., Han, M.F. & Hellmuth, A.E. Population of muscle satellite cells in relation to age and mitotic activity. Pathology 3, 223–243 (1971).
Shefer, G., Van de Mark, D.P., Richardson, J.B. & Yablonka-Reuveni, Z. Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev. Biol. 294, 50–66 (2006).
Conboy, I.M. & Rando, T.A. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev. Cell 3, 397–409 (2002).
Carlson, M.E., Hsu, M. & Conboy, I.M. Imbalance between pSmad3 and Notch induces CDK inhibitors in old muscle stem cells. Nature 454, 528–532 (2008).
Conboy, I.M., Conboy, M.J., Smythe, G.M. & Rando, T.A. Notch-mediated restoration of regenerative potential to aged muscle. Science 302, 1575–1577 (2003).
Brack, A.S. et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317, 807–810 (2007).
Brack, A.S. & Rando, T.A. Intrinsic changes and extrinsic influences of myogenic stem cell function during aging. Stem Cell Rev. 3, 226–237 (2007).
Sousa-Victor, P. et al. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature 506, 316–321 (2014).
Cosgrove, B.D. et al. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat. Med. 20, 255–264 (2014).
Bernet, J.D. et al. p38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice. Nat. Med. 20, 265–271 (2014).
Bentzinger, C.F. & Rudnicki, M.A. Rejuvenating aged muscle stem cells. Nat. Med. 20, 234–235 (2014).
Yablonka-Reuveni, Z., Seger, R. & Rivera, A.J. Fibroblast growth factor promotes recruitment of skeletal muscle satellite cells in young and old rats. J. Histochem. Cytochem. 47, 23–42 (1999).
Neal, A., Boldrin, L. & Morgan, J.E. The satellite cell in male and female, developing and adult mouse muscle: distinct stem cells for growth and regeneration. PLoS ONE 7, e37950 (2012).
Bosnakovski, D. et al. Prospective isolation of skeletal muscle stem cells with a Pax7 reporter. Stem Cells 26, 3194–3204 (2008).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550 (2005).
Conboy, I.M. et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760–764 (2005).
Toth, K.G. et al. IL-6 induced STAT3 signalling is associated with the proliferation of human muscle satellite cells following acute muscle damage. PLoS ONE 6, e17392 (2011).
Troy, A. et al. Coordination of satellite cell activation and self-renewal by Par-complex–dependent asymmetric activation of p38α/β MAPK. Cell Stem Cell 11, 541–553 (2012).
Pasut, A., Jones, A.E. & Rudnicki, M.A. Isolation and culture of individual myofibers and their satellite cells from adult skeletal muscle. J. Vis. Exp. e50074 (2013).
Cooper, R.N. et al. In vivo satellite cell activation via Myf5 and MyoD in regenerating mouse skeletal muscle. J. Cell Sci. 112, 2895–2901 (1999).
Uehara, Y., Mochizuki, M., Matsuno, K., Haino, T. & Asai, A. Novel high-throughput screening system for identifying STAT3–SH2 antagonists. Biochem. Biophys. Res. Commun. 380, 627–631 (2009).
Eriksen, K.W. et al. Constitutive STAT3-activation in Sezary syndrome: tyrphostin AG490 inhibits STAT3-activation, interleukin-2 receptor expression and growth of leukemic Sezary cells. Leukemia 15, 787–793 (2001).
Launay, T., Noirez, P., Butler-Browne, G. & Agbulut, O. Expression of slow myosin heavy chain during muscle regeneration is not always dependent on muscle innervation and calcineurin phosphatase activity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R1508–R1514 (2006).
Shuai, K. & Liu, B. Regulation of JAK-STAT signalling in the immune system. Nat. Rev. Immunol. 3, 900–911 (2003).
Megeney, L.A., Perry, R.L., LeCouter, J.E. & Rudnicki, M.A. bFGF and LIF signaling activates STAT3 in proliferating myoblasts. Dev. Genet. 19, 139–145 (1996).
Yang, Y. et al. STAT3 induces muscle stem cell differentiation by interaction with myoD. Cytokine 46, 137–141 (2009).
McKay, B.R. et al. Elevated SOCS3 and altered IL-6 signaling is associated with age-related human muscle stem cell dysfunction. Am. J. Physiol. Cell Physiol. 304, C717–C728 (2013).
Chakkalakal, J.V., Jones, K.M., Basson, M.A. & Brack, A.S. The aged niche disrupts muscle stem cell quiescence. Nature 490, 355–360 (2012).
Jang, Y.C., Sinha, M., Cerletti, M., Dall'Osso, C. & Wagers, A.J. Skeletal muscle stem cells: effects of aging and metabolism on muscle regenerative function. Cold Spring Harb. Symp. Quant. Biol. 76, 101–111 (2011).
Harrison, D.A. The Jak/STAT pathway. Cold Spring Harb. Perspect. Biol. 4, a011205 (2012).
Le Grand, F., Jones, A.E., Seale, V., Scime, A. & Rudnicki, M.A. Wnt7a activates the planar cell polarity pathway to drive the symmetric expansion of satellite stem cells. Cell Stem Cell 4, 535–547 (2009).
von Maltzahn, J., Bentzinger, C.F. & Rudnicki, M.A. Wnt7a-Fzd7 signalling directly activates the Akt/mTOR anabolic growth pathway in skeletal muscle. Nat. Cell Biol. 14, 186–191 (2012).
Bentzinger, C.F. et al. Fibronectin regulates Wnt7a signaling and satellite cell expansion. Cell Stem Cell 12, 75–87 (2013).
Tepass, U. The apical polarity protein network in Drosophila epithelial cells: regulation of polarity, junctions, morphogenesis, cell growth, and survival. Annu. Rev. Cell Dev. Biol. 28, 655–685 (2012).
McCaffrey, L.M., Montalbano, J., Mihai, C. & Macara, I.G. Loss of the Par3 polarity protein promotes breast tumorigenesis and metastasis. Cancer Cell 22, 601–614 (2012).
Price, F.D. et al. Canonical Wnt signaling induces a primitive endoderm metastable state in mouse embryonic stem cells. Stem Cells 31, 752–764 (2013).
Briguet, A., Courdier-Fruh, I., Foster, M., Meier, T. & Magyar, J.P. Histological parameters for the quantitative assessment of muscular dystrophy in the mdx-mouse. Neuromuscul. Disord. 14, 675–682 (2004).
Huang, W., Sherman, B.T. & Lempicki, R.A. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 37, 1–13 (2009).
Huang, W., Sherman, B.T. & Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 (2009).
Mootha, V.K. et al. PGC-1α–responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 34, 267–273 (2003).
Acknowledgements
We thank P. Oleynik for conducting cell sorting and providing guidance regarding FACS isolation and analysis. We thank J. Ritchie for mouse husbandry, A. Grayston for his help with myofibers counts and G. Palidwor for RMA normalization of microarray data sets and constructive discussions. We thank V. Soleimani for constructive discussions and access to unpublished microarray data. We thank M. Kyba, University of Minnesota, for the Pax7-ZsGreen mice. F.D.P. was supported by the Canadian Stem Cell Network and a Doctoral Research Award from the Canadian Institutes of Health Research. J.v.M. was supported by a grant from the German Research Foundation (MA-3975/2-1). C.F.B. was supported by the Swiss National Science Foundation. M.A.R. holds the Canada Research Chair in Molecular Genetics. These studies were carried out with support of grants to M.A.R. from the US National Institutes of Health (R01AR044031), the Canadian Institutes for Health Research (MOP-81288), the Stem Cell Network and the Ontario Ministry of Economic Development and Innovation.
Author information
Authors and Affiliations
Contributions
F.D.P. designed and carried out experiments, analyzed results and wrote the manuscript. J.v.M. designed and conducted experiments and analyzed results. C.F.B., N.A.D., H.Y. and N.C.C. conducted experiments and analyzed and interpreted data. D.H.W. conducted experiments. J.F. provided expertise in physiological analysis. M.A.R. designed experiments, analyzed results, wrote the manuscript and provided financial support.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 and Supplementary Table 1 (PDF 1639 kb)
Rights and permissions
About this article
Cite this article
Price, F., von Maltzahn, J., Bentzinger, C. et al. Inhibition of JAK-STAT signaling stimulates adult satellite cell function. Nat Med 20, 1174–1181 (2014). https://doi.org/10.1038/nm.3655
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3655