Abstract
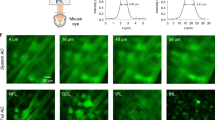
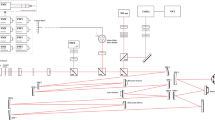
Two-photon excitation microscopy can image retinal molecular processes in vivo. Intrinsically fluorescent retinyl esters in subcellular structures called retinosomes are an integral part of the visual chromophore regeneration pathway. Fluorescent condensation products of all-trans-retinal accumulate in the eye with age and are also associated with age-related macular degeneration (AMD). Here, we report repetitive, dynamic imaging of these compounds in live mice through the pupil of the eye. By leveraging advanced adaptive optics, we developed a data acquisition algorithm that permitted the identification of retinosomes and condensation products in the retinal pigment epithelium by their characteristic localization, spectral properties and absence in genetically modified or drug-treated mice. This imaging approach has the potential to detect early molecular changes in retinoid metabolism that trigger light- and AMD-induced retinal defects and to assess the effectiveness of treatments for these conditions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Helmchen, F. & Denk, W. Deep tissue two-photon microscopy. Nat. Methods 2, 932–940 (2005).
Boettner, E.A. & Wolter, J.R. Transmission of the ocular media. Invest. Ophthalmol. Vis. Sci. 1, 776–783 (1962).
Imanishi, Y., Batten, M.L., Piston, D.W., Baehr, W. & Palczewski, K. Noninvasive two-photon imaging reveals retinyl ester storage structures in the eye. J. Cell Biol. 164, 373–383 (2004).
Imanishi, Y., Gerke, V. & Palczewski, K. Retinosomes: new insights into intracellular managing of hydrophobic substances in lipid bodies. J. Cell Biol. 166, 447–453 (2004).
Palczewska, G. et al. Noninvasive multiphoton fluorescence microscopy resolves retinol and retinal condensation products in mouse eyes. Nat. Med. 16, 1444–1449 (2010).
Denk, W., Strickler, J.H. & Webb, W.W. Two-photon laser scanning fluorescence microscopy. Science 248, 73–76 (1990).
Hunter, J.J. et al. Images of photoreceptors in living primate eyes using adaptive optics two-photon ophthalmoscopy. Biomed. Opt. Express 2, 139–148 (2010).
Godara, P., Dubis, A.M., Roorda, A., Duncan, J.L. & Carroll, J. Adaptive optics retinal imaging: emerging clinical applications. Optom. Vis. Sci. 87, 930–941 (2010).
Liang, J., Williams, D.R. & Miller, D.T. Supernormal vision and high-resolution retinal imaging through adaptive optics. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 14, 2884–2892 (1997).
Sharma, R. et al. In vivo two-photon imaging of the mouse retina. Biomed. Opt. Express 4, 1285–1293 (2013).
Rueckel, M., Mack-Bucher, J.A. & Denk, W. Adaptive wavefront correction in two-photon microscopy using coherence-gated wavefront sensing. Proc. Natl. Acad. Sci. USA 103, 17137–17142 (2006).
Huang, S., Heikal, A.A. & Webb, W.W. Two-photon fluorescence spectroscopy and microscopy of NAD(P)H and flavoprotein. Biophys. J. 82, 2811–2825 (2002).
Padayatti, P., Palczewska, G., Sun, W., Palczewski, K. & Salom, D. Imaging of protein crystals with two-photon microscopy. Biochemistry 51, 1625–1637 (2012).
Kiser, P.D., Golczak, M., Maeda, A. & Palczewski, K. Key enzymes of the retinoid (visual) cycle in vertebrate retina. Biochim. Biophys. Acta 1821, 137–151 (2012).
Palczewski, K. Chemistry and biology of vision. J. Biol. Chem. 287, 1612–1619 (2012).
von Lintig, J., Kiser, P.D., Golczak, M. & Palczewski, K. The biochemical and structural basis for trans-to-cis isomerization of retinoids in the chemistry of vision. Trends Biochem. Sci. 35, 400–410 (2010).
Sparrow, J.R., Wu, Y., Kim, C.Y. & Zhou, J. Phospholipid meets all-trans-retinal: the making of RPE bisretinoids. J. Lipid Res. 51, 247–261 (2010).
Maeda, A. et al. Primary amines protect against retinal degeneration in mouse models of retinopathies. Nat. Chem. Biol. 8, 170–178 (2011).
Maeda, T. et al. QLT091001, a 9-cis-retinal analog, is well-tolerated by retinas of mice with impaired visual cycles. Invest. Ophthalmol. Vis. Sci. 54, 455–466 (2013).
Golczak, M., Kuksa, V., Maeda, T., Moise, A.R. & Palczewski, K. Positively charged retinoids are potent and selective inhibitors of the trans-cis isomerization in the retinoid (visual) cycle. Proc. Natl. Acad. Sci. USA 102, 8162–8167 (2005).
Lin, B., Wang, S.W. & Masland, R.H. Retinal ganglion cell type, size, and spacing can be specified independent of homotypic dendritic contacts. Neuron 43, 475–485 (2004).
Euler, T. et al. Eyecup scope—optical recordings of light stimulus-evoked fluorescence signals in the retina. Pflugers Arch. 457, 1393–1414 (2009).
Bueno, J.M., Gualda, E.J. & Artal, P. Adaptive optics multiphoton microscopy to study ex vivo ocular tissues. J. Biomed. Opt. 15, 066004 (2010).
Helmstaedter, M. et al. Connectomic reconstruction of the inner plexiform layer in the mouse retina. Nature 500, 168–174 (2013).
Sun, W., Li, N. & He, S. Large-scale morphological survey of mouse retinal ganglion cells. J. Comp. Neurol. 451, 115–126 (2002).
Zipfel, W.R., Williams, R.M. & Webb, W.W. Nonlinear magic: multiphoton microscopy in the biosciences. Nat. Biotechnol. 21, 1369–1377 (2003).
Chan, F., Bradley, A., Wensel, T.G. & Wilson, J.H. Knock-in human rhodopsin-GFP fusions as mouse models for human disease and targets for gene therapy. Proc. Natl. Acad. Sci. USA 101, 9109–9114 (2004).
Spiess, E. et al. Two-photon excitation and emission spectra of the green fluorescent protein variants ECFP, EGFP and EYFP. J. Microsc. 217, 200–204 (2005).
Schmucker, C. & Schaeffel, F. A paraxial schematic eye model for the growing C57BL/6 mouse. Vision Res. 44, 1857–1867 (2004).
Katz, M.L. & Redmond, T.M. Effect of Rpe65 knockout on accumulation of lipofuscin fluorophores in the retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 42, 3023–3030 (2001).
Drabent, R., Bryl, K., Smyk, B. & Ulbrych, K. Retinyl palmitate in water environment. J. Photochem. Photobiol. B 37, 254–260 (1997).
Chen, C. et al. Reduction of all-trans retinal to all-trans retinol in the outer segments of frog and mouse rod photoreceptors. Biophys. J. 88, 2278–2287 (2005).
Moroni, L., Gellini, C., Salvi, P.R. & Schettino, V. Fluorescence of all-trans-retinal as a crystal and in a dense solution phase. J. Phys. Chem. A 104, 11063–11069 (2000).
Keilhauer, C.N. & Delori, F.C. Near-infrared autofluorescence imaging of the fundus: visualization of ocular melanin. Invest. Ophthalmol. Vis. Sci. 47, 3556–3564 (2006).
London, A., Benhar, I. & Schwartz, M. The retina as a window to the brain-from eye research to CNS disorders. Nat. Rev. Neurol. 9, 44–53 (2013).
Kocaoglu, O.P. et al. Imaging cone photoreceptors in three dimensions and in time using ultrahigh resolution optical coherence tomography with adaptive optics. Biomed. Opt. Express 2, 748–763 (2011).
Carroll, J. et al. The effect of cone opsin mutations on retinal structure and the integrity of the photoreceptor mosaic. Invest. Ophthalmol. Vis. Sci. 53, 8006–8015 (2012).
Delori, F.C., Webb, R.H., Sliney, D.H. & American National Standards, I. Maximum permissible exposures for ocular safety (ANSI 2000), with emphasis on ophthalmic devices. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 24, 1250–1265 (2007).
Geng, Y. et al. Adaptive optics retinal imaging in the living mouse eye. Biomed. Opt. Express 3, 715–734 (2012).
Xi, P., Andegeko, Y., Pestov, D., Lovozoy, V.V. & Dantus, M. Two-photon imaging using adaptive phase compensated ultrashort laser pulses. J. Biomed. Opt. 14, 014002 (2009).
Golczak, M. et al. Lecithin:retinol acyltransferase is responsible for amidation of retinylamine, a potent inhibitor of the retinoid cycle. J. Biol. Chem. 280, 42263–42273 (2005).
Maeda, A. et al. Effects of potent inhibitors of the retinoid cycle on visual function and photoreceptor protection from light damage in mice. Mol. Pharmacol. 70, 1220–1229 (2006).
Zhang, Y., Poonja, S. & Roorda, A. MEMS-based adaptive optics scanning laser ophthalmoscopy. Opt. Lett. 31, 1268–1270 (2006).
Remtulla, S. & Hallett, P.E. A schematic eye for the mouse, and comparisons with the rat. Vision Res. 25, 21–31 (1985).
Thibos, L.N. et al. Standards for reporting the optical aberrations of eyes. J. Refract. Surg. 18, S652–S660 (2002).
Sun, Y., Duthaler, S. & Nelson, B.J. Autofocusing in computer microscopy: selecting the optimal focus algorithm. Microsc. Res. Tech. 65, 139–149 (2004).
Vorontsov, M.A. & Sivokon, V.P. Stochastic parallel-gradient-descent technique for high-resolution wave-front phase-distortion correction. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 15, 2745–2758 (1998).
Acknowledgements
We thank M. Redmond (National Eye Institute of the US National Institutes of Health (NIH)) for Rpe65−/− mice and J. Wilson (Baylor College of Medicine) for GFP-rhodopsin mice. We also thank the team at Bioptigen for advice on preparation of mice for live animal imaging, D. Piston for stimulating discussion and L.T. Webster Jr. and members of K.P.'s laboratory and Polgenix's team for critical comments on the manuscript. Research reported in this publication was supported by the National Eye Institute of the NIH under award numbers R01EY008061, R01EY009339, R24EY021126 and P30EY11373 and by the National Institute on Aging of the NIH under award number R44AG043645. N.S.A. was supported by NIH institutional training grants 5T32EY007157 and 5T32DK007319.
Author information
Authors and Affiliations
Contributions
G.P. and K.P. conceived and directed the project. G.P., M.G., Z.D. and N.S.A. designed and conducted experiments, and generated software. G.P., N.S.A. and K.P. prepared the manuscript. J.J.H. and D.R.W. edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
G.P. and Z.D. are employees of Polgenix. K.P. is Chief Scientific Officer at Polgenix. K.P. is an inventor of the U.S. patent no. 7,706,863 and U.S. patent no. 8,346,345, whose values may be affected by this publication. The laboratories of J.J.H and D.R.W. received support from Polgenix.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 (PDF 990 kb)
Imaging retina at different depths.
50 images were obtained ex vivo along the z axis in an eye of a 2-month-old WT mouse. Details of different layers come into focus as the z-axis translation stage moves at even intervals. The first image shows the nerve fiber layer and the 50th image is just behind RPE. The optic disc is in the top right corner. (WMV 3205 kb)
Stochastic parallel gradient descent (SPGD) optimization of imaging Rpe65−/− mice.
Forty steps of optimization were conducted, and the corresponding forty images for the trajectory are shown. The 30th image is the one with the best normalized variance (shown in Fig. 4c). (WMV 6425 kb)
Rights and permissions
About this article
Cite this article
Palczewska, G., Dong, Z., Golczak, M. et al. Noninvasive two-photon microscopy imaging of mouse retina and retinal pigment epithelium through the pupil of the eye. Nat Med 20, 785–789 (2014). https://doi.org/10.1038/nm.3590
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3590
This article is cited by
-
Adaptive optics: principles and applications in ophthalmology
Eye (2021)
-
Adaptive optics two-photon microscopy enables near-diffraction-limited and functional retinal imaging in vivo
Light: Science & Applications (2020)
-
Evaluation of the Returned Electromagnetic Signal from Retro-reflectors in Turbid Media
Scientific Reports (2019)
-
Prenatal Zidovudine Treatment Modifies Early Development of Rat Osteoid – Confocal Microspectroscopy Analysis
Journal of Fluorescence (2019)
-
Light in diagnosis, therapy and surgery
Nature Biomedical Engineering (2017)