Abstract
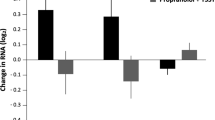
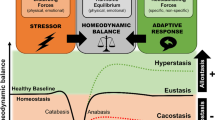
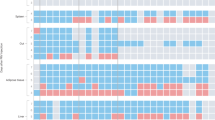
Exposure to psychosocial stress is a risk factor for many diseases, including atherosclerosis1,2. Although incompletely understood, interaction between the psyche and the immune system provides one potential mechanism linking stress and disease inception and progression. Known cross-talk between the brain and immune system includes the hypothalamic-pituitary-adrenal axis, which centrally drives glucocorticoid production in the adrenal cortex, and the sympathetic-adrenal-medullary axis, which controls stress-induced catecholamine release in support of the fight-or-flight reflex3,4. It remains unknown, however, whether chronic stress changes hematopoietic stem cell activity. Here we show that stress increases proliferation of these most primitive hematopoietic progenitors, giving rise to higher levels of disease-promoting inflammatory leukocytes. We found that chronic stress induced monocytosis and neutrophilia in humans. While investigating the source of leukocytosis in mice, we discovered that stress activates upstream hematopoietic stem cells. Under conditions of chronic variable stress in mice, sympathetic nerve fibers released surplus noradrenaline, which signaled bone marrow niche cells to decrease CXCL12 levels through the β3-adrenergic receptor. Consequently, hematopoietic stem cell proliferation was elevated, leading to an increased output of neutrophils and inflammatory monocytes. When atherosclerosis-prone Apoe−/− mice were subjected to chronic stress, accelerated hematopoiesis promoted plaque features associated with vulnerable lesions that cause myocardial infarction and stroke in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Black, P.H. The inflammatory response is an integral part of the stress response: implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain Behav. Immun. 17, 350–364 (2003).
Rosengren, A. et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet 364, 953–962 (2004).
Glaser, R. & Kiecolt-Glaser, J.K. Stress-induced immune dysfunction: implications for health. Nat. Rev. Immunol. 5, 243–251 (2005).
Powell, N.D. et al. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proc. Natl. Acad. Sci. USA 110, 16574–16579 (2013).
Cohen, S., Kamarck, T. & Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 24, 385–396 (1983).
Lesage, F.X. & Berjot, S. Validity of occupational stress assessment using a visual analogue scale. Occup. Med. (Lond.) 61, 434–436 (2011).
Schweizer, M.C., Henniger, M.S. & Sillaber, I. Chronic mild stress (CMS) in mice: of anhedonia, 'anomalous anxiolysis' and activity. PLoS ONE 4, e4326 (2009).
Nollet, M., Guisquet, A.M. & Belzung, C. Models of depression: unpredictable chronic mild stress in mice. Curr. Protoc. Pharmacol. 61, 5.65 (2013).
Yalcin, I., Aksu, F. & Belzung, C. Effects of desipramine and tramadol in a chronic mild stress model in mice are altered by yohimbine but not by pindolol. Eur. J. Pharmacol. 514, 165–174 (2005).
Kiel, M.J. et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121, 1109–1121 (2005).
Wilson, A. et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135, 1118–1129 (2008).
Szilvassy, S.J., Humphries, R.K., Lansdorp, P.M., Eaves, A.C. & Eaves, C.J. Quantitative assay for totipotent reconstituting hematopoietic stem cells by a competitive repopulation strategy. Proc. Natl. Acad. Sci. USA 87, 8736–8740 (1990).
Essers, M.A. et al. IFNα activates dormant haematopoietic stem cells in vivo. Nature 458, 904–908 (2009).
Baldridge, M.T., King, K.Y., Boles, N.C., Weksberg, D.C. & Goodell, M.A. Quiescent haematopoietic stem cells are activated by IFN-γ in response to chronic infection. Nature 465, 793–797 (2010).
Lo Celso, C., Lin, C.P. & Scadden, D.T. In vivo imaging of transplanted hematopoietic stem and progenitor cells in mouse calvarium bone marrow. Nat. Protoc. 6, 1–14 (2011).
Méndez-Ferrer, S., Lucas, D., Battista, M. & Frenette, P.S. Haematopoietic stem cell release is regulated by circadian oscillations. Nature 452, 442–447 (2008).
Spiegel, A. et al. Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nat. Immunol. 8, 1123–1131 (2007).
Lucas, D. et al. Chemotherapy-induced bone marrow nerve injury impairs hematopoietic regeneration. Nat. Med. 19, 695–703 (2013).
Zigmond, R.E. & Ben-Ari, Y. Electrical stimulation of preganglionic nerve increases tyrosine hydroxylase activity in sympathetic ganglia. Proc. Natl. Acad. Sci. USA 74, 3078–3080 (1977).
Morrison, S.J. & Scadden, D.T. The bone marrow niche for haematopoietic stem cells. Nature 505, 327–334 (2014).
Méndez-Ferrer, S. et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466, 829–834 (2010).
Ding, L. & Morrison, S.J. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 495, 231–235 (2013).
Eash, K.J., Means, J.M., White, D.W. & Link, D.C. CXCR4 is a key regulator of neutrophil release from the bone marrow under basal and stress granulopoiesis conditions. Blood 113, 4711–4719 (2009).
Tzeng, Y.S. et al. Loss of Cxcl12/Sdf-1 in adult mice decreases the quiescent state of hematopoietic stem/progenitor cells and alters the pattern of hematopoietic regeneration after myelosuppression. Blood 117, 429–439 (2011).
Nie, Y., Han, Y.C. & Zou, Y.R. CXCR4 is required for the quiescence of primitive hematopoietic cells. J. Exp. Med. 205, 777–783 (2008).
Libby, P., Ridker, P.M. & Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 473, 317–325 (2011).
Randolph, G.J. The fate of monocytes in atherosclerosis. J. Thromb. Haemost. 7 (suppl. 1), 28–30 (2009).
Rader, D.J. & Daugherty, A. Translating molecular discoveries into new therapies for atherosclerosis. Nature 451, 904–913 (2008).
Swirski, F.K. & Nahrendorf, M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 339, 161–166 (2013).
Moore, K.J. & Tabas, I. Macrophages in the pathogenesis of atherosclerosis. Cell 145, 341–355 (2011).
Libby, P. Mechanisms of acute coronary syndromes and their implications for therapy. N. Engl. J. Med. 368, 2004–2013 (2013).
Adamsson Eryd, S., Smith, J.G., Melander, O., Hedblad, B. & Engstrom, G. Incidence of coronary events and case fatality rate in relation to blood lymphocyte and neutrophil counts. Arterioscler. Thromb. Vasc. Biol. 32, 533–539 (2012).
Libby, P. Inflammation in atherosclerosis. Nature 420, 868–874 (2002).
Kaplan, J.R. et al. Social stress and atherosclerosis in normocholesterolemic monkeys. Science 220, 733–735 (1983).
Gu, H., Tang, C., Peng, K., Sun, H. & Yang, Y. Effects of chronic mild stress on the development of atherosclerosis and expression of toll-like receptor 4 signaling pathway in adolescent apolipoprotein E knockout mice. J. Biomed. Biotechnol. 2009, 613879 (2009).
Bernberg, E., Ulleryd, M.A., Johansson, M.E. & Bergstrom, G.M. Social disruption stress increases IL-6 levels and accelerates atherosclerosis in Apoe−/− mice. Atherosclerosis 221, 359–365 (2012).
Wilbert-Lampen, U. et al. Cardiovascular events during World Cup soccer. N. Engl. J. Med. 358, 475–483 (2008).
Wantha, S. et al. Neutrophil-derived cathelicidin promotes adhesion of classical monocytes. Circ. Res. 112, 792–801 (2013).
Weber, C. & Noels, H. Atherosclerosis: current pathogenesis and therapeutic options. Nat. Med. 17, 1410–1422 (2011).
Tall, A.R., Yvan-Charvet, L., Westerterp, M. & Murphy, A.J. Cholesterol efflux: a novel regulator of myelopoiesis and atherogenesis. Arterioscler. Thromb. Vasc. Biol. 32, 2547–2552 (2012).
Encinas, J.M et al. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell 8, 566–579 (2007).
Purton, L.E. & Scadden, D.T. Limiting factors in murine hematopoietic stem cell assays. Cell Stem Cell 1, 263–270 (2007).
Hu, Y. & Smyth, G.K. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J. Immunol. Methods 347, 70–78 (2009).
Dutta, P. et al. Myocardial infarction accelerates atherosclerosis. Nature 487, 325–329 (2012).
Kobayashi, M. & Srour, E.F. Regulation of murine hematopoietic stem cell quiescence by Dmtf1. Blood 118, 6562–6571 (2011).
Shi, C. et al. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating Toll-like receptor ligands. Immunity 34, 590–601 (2011).
Westerterp, M. et al. Regulation of hematopoietic stem and progenitor cell mobilization by cholesterol efflux pathways. Cell Stem Cell 11, 195–206 (2012).
Schepers, K. et al. Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche. Cell Stem Cell 13, 285–299 (2013).
Seimon, T.A. et al. Macrophage deficiency of p38α MAPK promotes apoptosis and plaque necrosis in advanced atherosclerotic lesions in mice. J. Clin. Invest. 119, 886–898 (2009).
Zaheer, A. et al. In vivo near-infrared fluorescence imaging of osteoblastic activity. Nat. Biotechnol. 19, 1148–1154 (2001).
Nahrendorf, M. et al. Hybrid PET-optical imaging using targeted probes. Proc. Natl. Acad. Sci. USA 107, 7910–7915 (2010).
Acknowledgements
We thank the team at the Center for Systems Biology Mouse Imaging Program, especially J. Truelove and D. Jeon, for help with imaging, M. Stein, I. Neudorfer and F. Meixner for help with the clinical study and L. Prickett-Rice, K. Folz-Donahue, M. Weglarz, M. Waring and A. Chicoine for assistance with cell sorting. We thank P. Frenette (Albert Einstein College of Medicine) and B. Lowell (Beth Israel Deaconess Medical Center) for providing Adrb3−/− mice and G. Enikolopov (Cold Spring Harbor Laboratory) for providing nestin-GFP mice. We thank the ICU team at the University Hospital Freiburg, Germany. This work was funded in part by US National Institutes of Health grants R01-HL114477, R01-HL117829 and R01-HL096576 (to M.N.) and grant HHSN268201000044C (to R.W.). T.H. and H.B.S. are funded by the Deutsche Forschungsgemeinschaft (HE-6382/1-1 to T.H. and SA1668/2-1 to H.B.S.).
Author information
Authors and Affiliations
Contributions
T.H. and H.B.S. performed experiments, collected, analyzed and discussed data and contributed to writing the manuscript. G.C., P.D., A.Z. and Y.I. performed experiments and collected, analyzed and discussed data. C.v.z.M., C.B., C.P.L., J.D., G.L.F., C.V., P.L., F.K.S. and R.W. conceived experiments and discussed results and strategy. M.N. managed and designed the study and wrote the manuscript, which was revised and approved by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–13 and Supplementary Tables 1 and 2 (PDF 3780 kb)
Rights and permissions
About this article
Cite this article
Heidt, T., Sager, H., Courties, G. et al. Chronic variable stress activates hematopoietic stem cells. Nat Med 20, 754–758 (2014). https://doi.org/10.1038/nm.3589
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3589
This article is cited by
-
Circulating myeloid-derived MMP8 in stress susceptibility and depression
Nature (2024)
-
Supra-additive effect of chronic inflammation and atherogenic dyslipidemia on developing type 2 diabetes among young adults: a prospective cohort study
Cardiovascular Diabetology (2023)
-
Neutrophil-derived catecholamines mediate negative stress effects on bone
Nature Communications (2023)
-
Traumatic brain injury stimulates sympathetic tone-mediated bone marrow myelopoiesis to favor fracture healing
Signal Transduction and Targeted Therapy (2023)
-
Central regulation of stress-evoked peripheral immune responses
Nature Reviews Neuroscience (2023)