Abstract
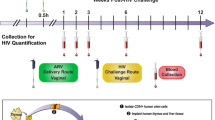
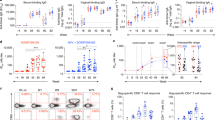
The vast majority of new HIV infections result from relatively inefficient transmission1,2 of the virus across mucosal surfaces during sexual intercourse3. A consequence of this inefficiency is that small numbers of transmitted founder viruses initiate most heterosexual infections4. This natural bottleneck to transmission has stimulated efforts to develop interventions that are aimed at blocking this step of the infection process5. Despite the promise of this strategy, clinical trials of preexposure prophylaxis have had limited degrees of success in humans, in part because of lack of adherence to the recommended preexposure treatment regimens6,7. In contrast, a number of existing vaccines elicit systemic immunity that protects against mucosal infections, such as the vaccines for influenza8 and human papilloma virus9. We recently demonstrated the ability of vectored immunoprophylaxis (VIP) to prevent intravenous transmission of HIV in humanized mice using broadly neutralizing antibodies10. Here we demonstrate that VIP is capable of protecting humanized mice from intravenous as well as vaginal challenge with diverse HIV strains despite repeated exposures. Moreover, animals receiving VIP that expresses a modified VRC07 antibody were completely resistant to repetitive intravaginal challenge by a heterosexually transmitted founder HIV strain11, suggesting that VIP may be effective in preventing vaginal transmission of HIV between humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Gray, R.H. et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1–discordant couples in Rakai, Uganda. Lancet 357, 1149–1153 (2001).
Pilcher, C.D. et al. Brief but efficient: acute HIV infection and the sexual transmission of HIV. J. Infect. Dis. 189, 1785–1792 (2004).
Kilmarx, P.H. Global epidemiology of HIV. Curr. Opin. HIV AIDS 4, 240–246 (2009).
Salazar-Gonzalez, J.F. et al. Deciphering human immunodeficiency virus type 1 transmission and early envelope diversification by single-genome amplification and sequencing. J. Virol. 82, 3952–3970 (2008).
Abdool Karim, Q. et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 329, 1168–1174 (2010).
Van Damme, L. et al. Preexposure prophylaxis for HIV infection among African women. N. Engl. J. Med. 367, 411–422 (2012).
Marrazzo, M.J. et al. Pre-exposure prophylaxis for HIV in women: Daily oral tenofovir, oral tenofovir/emtricitabine, or vaginal tenofovir gel in the VOICE study (MTN-003). 20th Conference on Retroviruses and Opportunistic Infections http://www.avac.org/ht/a/GetDocumentAction/i/49307 (2013).
Osterholm, M.T., Kelley, N.S., Sommer, A. & Belongia, E.A. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect. Dis. 12, 36–44 (2011).
Romanowski, B. Long term protection against cervical infection with the human papillomavirus: review of currently available vaccines. Hum. Vaccin. 7, 161–169 (2011).
Balazs, A.B. et al. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature 481, 81–84 (2011).
Ochsenbauer, C. et al. Generation of transmitted/founder HIV-1 infectious molecular clones and characterization of their replication capacity in CD4 T-lymphocytes and monocyte-derived macrophages. J. Virol. 86, 2715–2728 (2012).
Burton, D.R., Poignard, P., Stanfield, R.L. & Wilson, I.A. Broadly neutralizing antibodies present new prospects to counter highly antigenically diverse viruses. Science 337, 183–186 (2012).
Johnson, P.R. et al. Vector-mediated gene transfer engenders long-lived neutralizing activity and protection against SIV infection in monkeys. Nat. Med. 15, 901–906 (2009).
Koyanagi, Y. et al. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science 236, 819–822 (1987).
Poignard, P. et al. Neutralizing antibodies have limited effects on the control of established HIV-1 infection in vivo. Immunity 10, 431–438 (1999).
Zhou, T. et al. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science 329, 811–817 (2010).
Sun, Z. et al. Intrarectal transmission, systemic infection, and CD4+ T cell depletion in humanized mice infected with HIV-1. J. Exp. Med. 204, 705–714 (2007).
Denton, P.W. et al. Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med. 5, e16 (2008).
Hessell, A.J. et al. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat. Med. 15, 951–954 (2009).
Walker, L.M. et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477, 466–470 (2011).
Wu, X. et al. Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science 16, 1593–1602 (2011).
Scheid, J.F. et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science 333, 1633–1637 (2011).
Diskin, R. et al. Increasing the potency and breadth of an HIV antibody by using structure-based rational design. Science 334, 1289–1293 (2011).
Kwon, Y. et al. Structure-guided modification and optimization of antibody VRC07. Retrovirology 9, O34 (2012).
Parrish, N.F. et al. Phenotypic properties of transmitted founder HIV-1. Proc. Natl. Acad. Sci. USA 110, 6626–6633 (2013).
Lim, S.G. et al. Loss of mucosal CD4 lymphocytes is an early feature of HIV infection. Clin. Exp. Immunol. 92, 448–454 (1993).
Melkus, M.W. et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat. Med. 12, 1316–1322 (2006).
Acknowledgements
We acknowledge G. Nabel (Sanofi-Pasteur) and J. Mascola (US National Institutes of Health (NIH) Vaccine Research Center) for VRC01, VRC-PG04, VRC07 and VRC07G54W expression plasmids and proteins, D. Burton (Scripps) for b12, PG9, PGT121 and PGT128 expression plasmids, M. Nussenzweig (Rockefeller) for 3BNC117 and 12A12 expression plasmids and P. Bjorkman (California Institute of Technology) for the NIH45-46W expression plasmid. We also thank the Caltech Protein Expression Center for providing purified antibodies. The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID), NIH: pYK-JRCSF from I.S.Y. Chen and Y. Koyanagi, pREJO.c/2864 from J. Kappes and C. Ochsenbauer and TZM-bl cells from J. Kappes and X. Wu. We thank J. Kim, D. Majumdar, M. Mann and A. So for their helpful comments and other members of the Baltimore lab, as well as R. Cortado and S. Shimizu in the An lab, for their assistance in carrying out this work. Preparation of human CD34+ cells, tissue procurement and BLT mice were supported by the UCLA Center for AIDS Research (CFAR) AI028697. A.B.B. is supported by the NIAID Career Transition Award 1K22AI102769. D.S.R. was a Sidney Kimmel Scholar supported by the Sidney Kimmel Foundation for Cancer Research (Translational Award SKF-11-013) and is supported by career development award 1K08CA133521 from the NIH. D.S.A. is supported by NIAID grant 1R01AI100652-01A1. This project was supported by the NIH (HHSN266200500035C) through a contract from the NIAID and by the Joint Center for Translational Medicine.
Author information
Authors and Affiliations
Contributions
A.B.B. and D.B. conceived the study. A.B.B. designed the experiments. D.S.A. offered suggestions for the experiments and provided the BLT humanized mice. A.B.B., Y.O., C.M.H., J.C. and S.M.N. carried out experiments. A.B.B., Y.O., C.M.H., J.C. and S.M.N. analyzed the data. D.S.R. performed immunohistochemistry and analysis. A.B.B. and D.B. wrote the paper with contributions from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 (PDF 12230 kb)
Rights and permissions
About this article
Cite this article
Balazs, A., Ouyang, Y., Hong, C. et al. Vectored immunoprophylaxis protects humanized mice from mucosal HIV transmission. Nat Med 20, 296–300 (2014). https://doi.org/10.1038/nm.3471
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3471