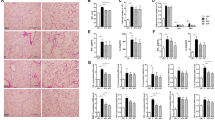
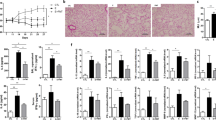
Abstract
Metabolites from intestinal microbiota are key determinants of host-microbe mutualism and, consequently, the health or disease of the intestinal tract. However, whether such host-microbe crosstalk influences inflammation in peripheral tissues, such as the lung, is poorly understood. We found that dietary fermentable fiber content changed the composition of the gut and lung microbiota, in particular by altering the ratio of Firmicutes to Bacteroidetes. The gut microbiota metabolized the fiber, consequently increasing the concentration of circulating short-chain fatty acids (SCFAs). Mice fed a high-fiber diet had increased circulating levels of SCFAs and were protected against allergic inflammation in the lung, whereas a low-fiber diet decreased levels of SCFAs and increased allergic airway disease. Treatment of mice with the SCFA propionate led to alterations in bone marrow hematopoiesis that were characterized by enhanced generation of macrophage and dendritic cell (DC) precursors and subsequent seeding of the lungs by DCs with high phagocytic capacity but an impaired ability to promote T helper type 2 (TH2) cell effector function. The effects of propionate on allergic inflammation were dependent on G protein–coupled receptor 41 (GPR41, also called free fatty acid receptor 3 or FFAR3), but not GPR43 (also called free fatty acid receptor 2 or FFAR2). Our results show that dietary fermentable fiber and SCFAs can shape the immunological environment in the lung and influence the severity of allergic inflammation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Devereux, G. The increase in the prevalence of asthma and allergy: food for thought. Nat. Rev. Immunol. 6, 869–874 (2006).
Nakaji, S. et al. Trends in dietary fiber intake in Japan over the last century. Eur. J. Nutr. 41, 222–227 (2002).
Roediger, W.E. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut 21, 793–798 (1980).
Binder, H.J. & Mehta, P. Short-chain fatty acids stimulate active sodium and chloride absorption in vitro in the rat distal colon. Gastroenterology 96, 989–996 (1989).
Kaneko, T., Mori, H., Iwata, M. & Meguro, S. Growth stimulator for bifidobacteria produced by Propionibacterium freudenreichii and several intestinal bacteria. J. Dairy Sci. 77, 393–404 (1994).
Xie, S., Liu, J., Li, L. & Qiao, C. Biodegradation of malathion by Acinetobacter johnsonii MA19 and optimization of cometabolism substrates. J. Environ. Sci. (China) 21, 76–82 (2009).
Flint, H.J., Bayer, E.A., Rincon, M.T., Lamed, R. & White, B.A. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat. Rev. Microbiol. 6, 121–131 (2008).
Le Poul, E. et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 278, 25481–25489 (2003).
Maslowski, K.M. et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461, 1282–1286 (2009).
Sina, C. et al. G protein-coupled receptor 43 is essential for neutrophil recruitment during intestinal inflammation. J. Immunol. 183, 7514–7522 (2009).
Aoyama, M., Kotani, J. & Usami, M. Butyrate and propionate induced activated or non-activated neutrophil apoptosis via HDAC inhibitor activity but without activating GPR-41/GPR-43 pathways. Nutrition 26, 653–661 (2010).
Jansen, M.S. et al. Short-chain fatty acids enhance nuclear receptor activity through mitogen-activated protein kinase activation and histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 101, 7199–7204 (2004).
Kim, H.J. et al. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action. J. Pharmacol. Exp. Ther. 321, 892–901 (2007).
Cummings, J.H., Hill, M.J., Bone, E.S., Branch, W.J. & Jenkins, D.J. The effect of meat protein and dietary fiber on colonic function and metabolism. II. Bacterial metabolites in feces and urine. Am. J. Clin. Nutr. 32, 2094–2101 (1979).
Cummings, J.H., Pomare, E.W., Branch, W.J., Naylor, C.P. & Macfarlane, G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28, 1221–1227 (1987).
Greger, J.L. Nondigestible carbohydrates and mineral bioavailability. J. Nutr. 129, 1434S–1435S (1999).
Riedl, J., Linseisen, J., Hoffmann, J. & Wolfram, G. Some dietary fibers reduce the absorption of carotenoids in women. J. Nutr. 129, 2170–2176 (1999).
Scholz-Ahrens, K.E. & Schrezenmeir, J. Inulin and oligofructose and mineral metabolism: the evidence from animal trials. J. Nutr. 137, 2513S–2523S (2007).
Hill, D.A. et al. Metagenomic analyses reveal antibiotic-induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis. Mucosal Immunol. 3, 148–158 (2010).
Herbst, T. et al. Dysregulation of allergic airway inflammation in the absence of microbial colonization. Am. J. Respir. Crit. Care Med. 184, 198–205 (2011).
Olszak, T. et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science 336, 489–493 (2012).
Hill, D.A. et al. Commensal bacteria-derived signals regulate basophil hematopoiesis and allergic inflammation. Nat. Med. 18, 538–546 (2012).
Russell, S.L. et al. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep. 13, 440–447 (2012).
Noverr, M.C., Falkowski, N.R., McDonald, R.A., McKenzie, A.N. & Huffnagle, G.B. Development of allergic airway disease in mice following antibiotic therapy and fungal microbiota increase: role of host genetics, antigen, and interleukin-13. Infect. Immun. 73, 30–38 (2005).
Noverr, M.C., Noggle, R.M., Toews, G.B. & Huffnagle, G.B. Role of antibiotics and fungal microbiota in driving pulmonary allergic responses. Infect. Immun. 72, 4996–5003 (2004).
Turnbaugh, P.J., Backhed, F., Fulton, L. & Gordon, J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 3, 213–223 (2008).
De Filippo, C. et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 107, 14691–14696 (2010).
Maslowski, K.M. & Mackay, C.R. Diet, gut microbiota and immune responses. Nat. Immunol. 12, 5–9 (2011).
Ulven, T. Short-chain free fatty acid receptors FFA2/GPR43 and FFA3/GPR41 as new potential therapeutic targets. Front. Endocrinol. (Lausanne) 3, 111 (2012).
Lambrecht, B.N. & Hammad, H. Taking our breath away: dendritic cells in the pathogenesis of asthma. Nat. Rev. Immunol. 3, 994–1003 (2003).
Lambrecht, B.N., Salomon, B., Klatzmann, D. & Pauwels, R.A. Dendritic cells are required for the development of chronic eosinophilic airway inflammation in response to inhaled antigen in sensitized mice. J. Immunol. 160, 4090–4097 (1998).
Plantinga, M. et al. Conventional and monocyte-derived CD11b+ dendritic cells initiate and maintain T helper 2 cell–mediated immunity to house dust mite allergen. Immunity 38, 322–335 (2013).
Liu, K. et al. In vivo analysis of dendritic cell development and homeostasis. Science 324, 392–397 (2009).
Lambrecht, B.N. & Hammad, H. Lung dendritic cells in respiratory viral infection and asthma: from protection to immunopathology. Annu. Rev. Immunol. 30, 243–270 (2012).
Erb-Downward, J.R. et al. Analysis of the lung microbiome in the “healthy” smoker and in COPD. PLoS ONE 6, e16384 (2011).
Hilty, M. et al. Disordered microbial communities in asthmatic airways. PLoS ONE 5, e8578 (2010).
Naik, S. et al. Compartmentalized control of skin immunity by resident commensals. Science 337, 1115–1119 (2012).
McNeil, N.I. The contribution of the large intestine to energy supplies in man. Am. J. Clin. Nutr. 39, 338–342 (1984).
Vinolo, M.A., Rodrigues, H.G., Nachbar, R.T. & Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 3, 858–876 (2011).
Smith, P.M. et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573 (2013).
Arpaia, N. et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455 (2013).
Blainey, P.C., Milla, C.E., Cornfield, D.N. & Quake, S.R. Quantitative analysis of the human airway microbial ecology reveals a pervasive signature for cystic fibrosis. Sci. Transl. Med. 4, 153ra130 (2012).
Madan, J.C. et al. Serial analysis of the gut and respiratory microbiome in cystic fibrosis in infancy: interaction between intestinal and respiratory tracts and impact of nutritional exposures. mBio 3, e00251–12 (2012).
Acknowledgements
This work has been supported by the Swiss National Science Foundation grant 310030.130029 awarded to B.J.M. We thank R. Driscoll and the Fondation Placide Nicod for support and A. Genevaz, B. Berger and E. Rezzonico for scientific assistance related to microbiota analysis. B.J.M. is a Cloetta Medical Research Fellow.
Author information
Authors and Affiliations
Contributions
B.J.M. conceived the study. B.J.M. and A.T. designed the study. A.T., E.S.G., K.Y. and A.K.S. performed experiments. A.T. and E.S.G. analyzed data. N.S. performed SCFA analysis. C.N.-B., A.T. and B.J.M. performed microbiota analysis. T.J. provided Ffar3−/− and Ffar2−/− mice. T.J., C.B., N.L.H., L.P.N., E.S.G., A.T. and B.J.M. provided critical analysis and discussions. B.J.M. and A.T. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
C.N.-B., C.B. and N.S. are employed by Nestec Ltd, Switzerland. T.J. is employed by Novartis Institutes for Biomedical Research.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 1650 kb)
Rights and permissions
About this article
Cite this article
Trompette, A., Gollwitzer, E., Yadava, K. et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 20, 159–166 (2014). https://doi.org/10.1038/nm.3444
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3444