Abstract
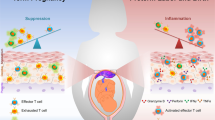
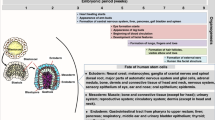
An improved mechanistic understanding of the adaptational processes mounted during mammalian reproduction is emerging. Intricate pathways occurring at the fetomaternal interface, such as the formation of a functional synapse between invading fetal trophoblast cells, and the involvement of various maternal immune cell subsets and epigenetically modified decidual stromal cells have now been identified. These complex pathways synergistically create a tolerogenic niche in which the semiallogeneic fetus can develop. New insights into fetomaternal immune cross-talk may help us to understand the pathogenesis of pregnancy complications as well as poor postnatal health. Moreover, the effects of maternal immune adaptation to pregnancy on autoimmune disease activity are becoming increasingly evident. Thus, insights into fetomaternal immune cross-talk not only advance our understanding of pregnancy-related complications but also may be informative on how immune tolerance can be modulated in clinical settings outside the context of reproduction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Owen, R.D. Immunogenetic consequences of vascular anastomoses between bovine twins. Science 102, 400–401 (1945).
Medawar, P.B. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp. Soc. Exp. Biol. 7, 320–338 (1953).
Billingham, R.E., Brent, L. & Medawar, P.B. Actively acquired tolerance of foreign cells. Nature 172, 603–606 (1953).
Elliot, M.G. & Crespi, B.J. Placental invasiveness mediates the evolution of hybrid inviability in mammals. Am. Nat. 168, 114–120 (2006).
Moffett, A. & Loke, C. Immunology of placentation in eutherian mammals. Nat. Rev. Immunol. 6, 584–594 (2006).
Lin, H. et al. Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J. Immunol. 151, 4562–4573 (1993).
Chaouat, G. The Th1/Th2 paradigm: still important in pregnancy? Semin. Immunopathol. 29, 95–113 (2007).
Kim, M.R. et al. Progesterone-dependent release of transforming growth factor-β1 from epithelial cells enhances the endometrial decidualization by turning on the Smad signalling in stromal cells. Mol. Hum. Reprod. 11, 801–808 (2005).
Dunn, C.L., Kelly, R.W. & Critchley, H.O. Decidualization of the human endometrial stromal cell: an enigmatic transformation. Reprod. Biomed. Online 7, 151–161 (2003).
Norwitz, E.R., Schust, D.J. & Fisher, S.J. Implantation and the survival of early pregnancy. N. Engl. J. Med. 345, 1400–1408 (2001).
Ren, L., Liu, Y.Q., Zhou, W.H. & Zhang, Y.Z. Trophoblast-derived chemokine CXCL12 promotes CXCR4 expression and invasion of human first-trimester decidual stromal cells. Hum. Reprod. 27, 366–374 (2012).
Aplin, J.D., Charlton, A.K. & Ayad, S. An immunohistochemical study of human endometrial extracellular matrix during the menstrual cycle and first trimester of pregnancy. Cell Tissue Res. 253, 231–240 (1988).
Lockwood, C.J. et al. Decidual cell-expressed tissue factor in human pregnancy and its involvement in hemostasis and preeclampsia-related angiogenesis. Ann. NY Acad. Sci. 1127, 67–72 (2008).
Segerer, S.E. et al. MIC-1 (a multifunctional modulator of dendritic cell phenotype and function) is produced by decidual stromal cells and trophoblasts. Hum. Reprod. 27, 200–209 (2012).
Cavanagh, P.C. et al. Gonadotropin-releasing hormone-regulated chemokine expression in human placentation. Am. J. Physiol. Cell Physiol. 297, C17–C27 (2009).
Zhou, W.H., Du, M.R., Dong, L., Yu, J. & Li, D.J. Chemokine CXCL12 promotes the cross-talk between trophoblasts and decidual stromal cells in human first-trimester pregnancy. Hum. Reprod. 23, 2669–2679 (2008).
Wu, X. et al. Human first-trimester trophoblast cells recruit CD56brightCD16− NK cells into decidua by way of expressing and secreting of CXCL12/stromal cell–derived factor 1. J. Immunol. 175, 61–68 (2005).
Hanna, J. et al. CXCL12 expression by invasive trophoblasts induces the specific migration of CD16− human natural killer cells. Blood 102, 1569–1577 (2003).
Pearce, W.J. Multifunctional angiogenic factors: add GnRH to the list. Focus on “Gonadotropin-releasing hormone-regulated chemokine expression in human placentation”. Am. J. Physiol. Cell Physiol. 297, C4–C5 (2009).
Red-Horse, K., Drake, P.M., Gunn, M.D. & Fisher, S.J. Chemokine ligand and receptor expression in the pregnant uterus: reciprocal patterns in complementary cell subsets suggest functional roles. Am. J. Pathol. 159, 2199–2213 (2001).
Moffett-King, A. Natural killer cells and pregnancy. Nat. Rev. Immunol. 2, 656–663 (2002).
García-López, M.A. et al. CXCR3 chemokine receptor distribution in normal and inflamed tissues: expression on activated lymphocytes, endothelial cells, and dendritic cells. Lab. Invest. 81, 409–418 (2001).
Bromley, S.K., Mempel, T.R. & Luster, A.D. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat. Immunol. 9, 970–980 (2008).
Vacca, P. et al. CD34+ hematopoietic precursors are present in human decidua and differentiate into natural killer cells upon interaction with stromal cells. Proc. Natl. Acad. Sci. USA 108, 2402–2407 (2011).
Keskin, D.B. et al. TGFβ promotes conversion of CD16+ peripheral blood NK cells into CD16- NK cells with similarities to decidual NK cells. Proc. Natl. Acad. Sci. USA 104, 3378–3383 (2007).
Croy, B.A., van den Heuvel, M.J., Borzychowski, A.M. & Tayade, C. Uterine natural killer cells: a specialized differentiation regulated by ovarian hormones. Immunol. Rev. 214, 161–185 (2006).
Chantakru, S., Kuziel, W.A., Maeda, N. & Croy, B.A. A study on the density and distribution of uterine Natural Killer cells at mid pregnancy in mice genetically-ablated for CCR2, CCR 5 and the CCR5 receptor ligand, MIP-1α. J. Reprod. Immunol. 49, 33–47 (2001).
Kruse, A., Merchant, M.J., Hallmann, R. & Butcher, E.C. Evidence of specialized leukocyte-vascular homing interactions at the maternal/fetal interface. Eur. J. Immunol. 29, 1116–1126 (1999).
King, A. et al. HLA-E is expressed on trophoblast and interacts with CD94/NKG2 receptors on decidual NK cells. Eur. J. Immunol. 30, 1623–1631 (2000).
Li, C., Houser, B.L., Nicotra, M.L. & Strominger, J.L. HLA-G homodimer-induced cytokine secretion through HLA-G receptors on human decidual macrophages and natural killer cells. Proc. Natl. Acad. Sci. USA 106, 5767–5772 (2009).
van der Meer, A. et al. Membrane-bound HLA-G activates proliferation and interferon-γ production by uterine natural killer cells. Mol. Hum. Reprod. 10, 189–195 (2004).
LeMaoult, J., Krawice-Radanne, I., Dausset, J. & Carosella, E.D. HLA-G1–expressing antigen-presenting cells induce immunosuppressive CD4+ T cells. Proc. Natl. Acad. Sci. USA 101, 7064–7069 (2004).
King, A. et al. Surface expression of HLA-C antigen by human extravillous trophoblast. Placenta 21, 376–387 (2000).
Chazara, O., Xiong, S. & Moffett, A. Maternal KIR and fetal HLA-C: a fine balance. J. Leukoc. Biol. 90, 703–716 (2011).
Uhrberg, M. et al. Human diversity in killer cell inhibitory receptor genes. Immunity 7, 753–763 (1997).
Parham, P. MHC class I molecules and KIRs in human history, health and survival. Nat. Rev. Immunol. 5, 201–214 (2005).
Sharkey, A.M. et al. Killer Ig-like receptor expression in uterine NK cells is biased toward recognition of HLA-C and alters with gestational age. J. Immunol. 181, 39–46 (2008).
Lyall, F., Bulmer, J.N., Kelly, H., Duffie, E. & Robson, S.C. Human trophoblast invasion and spiral artery transformation: the role of nitric oxide. Am. J. Pathol. 154, 1105–1114 (1999).
Pijnenborg, R., Vercruysse, L. & Hanssens, M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta 27, 939–958 (2006).
Zhang, J. et al. Unusual timing of CD127 expression by mouse uterine natural killer cells. J. Leukoc. Biol. 91, 417–426 (2012).
Karimi, K. et al. Regulation of pregnancy maintenance and fetal survival in mice by CD27low mature NK cells. J. Mol. Med. 90, 1047–1057 (2012).
Huang, Y., Zhu, X.Y., Du, M.R. & Li, D.J. Human trophoblasts recruited T lymphocytes and monocytes into decidua by secretion of chemokine CXCL16 and interaction with CXCR6 in the first-trimester pregnancy. J. Immunol. 180, 2367–2375 (2008).
Kämmerer, U. et al. Human decidua contains potent immunostimulatory CD83+ dendritic cells. J. Am. J. Pathol. 157, 159–169 (2000).
Blois, S.M. et al. Lineage, maturity, and phenotype of uterine murine dendritic cells throughout gestation indicate a protective role in maintaining pregnancy. Biol. Reprod. 70, 1018–1023 (2004).
Steinman, R.M. Decisions about dendritic cells: past, present, and future. Annu. Rev. Immunol. 30, 1–22 (2012).
Blois, S.M. et al. A pivotal role for galectin-1 in fetomaternal tolerance. Nat. Med. 13, 1450–1457 (2007).
Kämmerer, U. et al. Unique appearance of proliferating antigen-presenting cells expressing DC-SIGN (CD209) in the decidua of early human pregnancy. Am. J. Pathol. 162, 887–896 (2003).
Ilarregui, J.M. et al. Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1–driven immunoregulatory circuit involving interleukin 27 and interleukin 10. Nat. Immunol. 10, 981–991 (2009).
Ferreira, G.B. et al. Differential protein pathways in 1,25-dihydroxyvitamin d(3) and dexamethasone modulated tolerogenic human dendritic cells. J. Proteome Res. 11, 941–971 (2012).
Bootcov, M.R. et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-β superfamily. Proc. Natl. Acad. Sci. USA 94, 11514–11519 (1997).
Penna, G. et al. 1,25-Dihydroxyvitamin D3 selectively modulates tolerogenic properties in myeloid but not plasmacytoid dendritic cells. J. Immunol. 178, 145–153 (2007).
Dotan, I. et al. CXCL12 Is a constitutive and inflammatory chemokine in the intestinal immune system. Inflamm. Bowel Dis. 16, 583–592 (2010).
Mjösberg, J., Berg, G., Jenmalm, M.C. & Ernerudh, J. FOXP3+ regulatory T cells and T helper 1, T helper 2, and T helper 17 cells in human early pregnancy decidua. Biol. Reprod. 82, 698–705 (2010).
Nancy, P. et al. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science 336, 1317–1321 (2012).
Grégoire, C. et al. The trafficking of natural killer cells. Immunol. Rev. 220, 169–182 (2007).
Hanna, J. et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat. Med. 12, 1065–1074 (2006).
Erlebacher, A., Vencato, D., Price, K.A., Zhang, D. & Glimcher, L.H. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J. Clin. Invest. 117, 1399–1411 (2007).
Collins, M.K., Tay, C.S. & Erlebacher, A. Dendritic cell entrapment within the pregnant uterus inhibits immune surveillance of the maternal/fetal interface in mice. J. Clin. Invest. 119, 2062–2073 (2009).
Moldenhauer, L.M., Keenihan, S.N., Hayball, J.D. & Robertson, S.A. GM-CSF is an essential regulator of T cell activation competence in uterine dendritic cells during early pregnancy in mice. J. Immunol. 185, 7085–7096 (2010).
Moldenhauer, L.M., Hayball, J.D. & Robertson, S.A. Utilising T cell receptor transgenic mice to define mechanisms of maternal T cell tolerance in pregnancy. J. Reprod. Immunol. 87, 1–13 (2010).
Böckle, B.C., Sölder, E., Kind, S., Romani, N. & Sepp, N.T. DC-sign+ CD163+ macrophages expressing hyaluronan receptor LYVE-1 are located within chorion villi of the placenta. Placenta 29, 187–192 (2008).
Red-Horse, K. et al. Cytotrophoblast induction of arterial apoptosis and lymphangiogenesis in an in vivo model of human placentation. J. Clin. Invest. 116, 2643–2652 (2006).
Redman, C.W. et al. Does size matter? Placental debris and the pathophysiology of pre-eclampsia. Placenta 33, S48–S54 (2012).
Baban, B. et al. Indoleamine 2,3-dioxygenase expression is restricted to fetal trophoblast giant cells during murine gestation and is maternal genome specific. J. Reprod. Immunol. 61, 67–77 (2004).
Hunt, J.S., Vassmer, D., Ferguson, T.A. & Miller, L. Fas ligand is positioned in mouse uterus and placenta to prevent trafficking of activated leukocytes between the mother and the conceptus. J. Immunol. 158, 4122–4128 (1997).
Bai, X. et al. A placental protective role for trophoblast-derived TNF-related apoptosis-inducing ligand (TRAIL). Placenta 30, 855–860 (2009).
Nagamatsu, T., Schust, D.J., Sugimoto, J. & Barrier, B.F. Human decidual stromal cells suppress cytokine secretion by allogenic CD4+ T cells via PD-1 ligand interactions. Hum. Reprod. 24, 3160–3171 (2009).
Petroff, M.G., Kharatyan, E., Torry, D.S. & Holets, L. The immunomodulatory proteins B7-DC, B7–H2, and B7–H3 are differentially expressed across gestation in the human placenta. Am. J. Pathol. 167, 465–473 (2005).
Taglauer, E.S., Yankee, T.M. & Petroff, M.G. Maternal PD-1 regulates accumulation of fetal antigen-specific CD8+ T cells in pregnancy. J. Reprod. Immunol. 80, 12–21 (2009).
Chaouat, G. & Clark, D.A. FAS/FAS ligand interaction at the placental interface is not required for the success of allogeneic pregnancy in anti-paternal MHC preimmunized mice. Am. J. Reprod. Immunol. 45, 108–115 (2001).
Guleria, I. et al. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J. Exp. Med. 202, 231–237 (2005).
Zhao, J.X., Zeng, Y.Y. & Liu, Y. Fetal alloantigen is responsible for the expansion of the CD4+ CD25+ regulatory T cell pool during pregnancy. J. Reprod. Immunol. 75, 71–81 (2007).
Thuere, C. et al. Kinetics of regulatory T cells during murine pregnancy. Am. J. Reprod. Immunol. 58, 514–523 (2007).
Robertson, S.A., Guerin, L.R., Moldenhauer, L.M. & Hayball, J.D. Activating T regulatory cells for tolerance in early pregnancy - the contribution of seminal fluid. J. Reprod. Immunol. 83, 109–116 (2009).
Sasaki, Y. et al. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol. Hum. Reprod. 10, 347–353 (2004).
Aluvihare, V.R., Kallikourdis, M. & Betz, A.G. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 5, 266–271 (2004).
Mjösberg, J. et al. Systemic reduction of functionally suppressive CD4dimCD25highFoxp3+ Tregs in human second trimester pregnancy is induced by progesterone and 17beta-estradiol. J. Immunol. 183, 759–769 (2009).
Kallikourdis, M., Andersen, K.G., Welch, K.A. & Betz, A.G. Alloantigen-enhanced accumulation of CCR5+ 'effector' regulatory T cells in the gravid uterus. Proc. Natl. Acad. Sci. USA 104, 594–599 (2007).
Rowe, J.H., Ertelt, J.M., Xin, L. & Way, S.S. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature 490, 102–106 (2012).
Zinaman, M.J., O'Connor, J., Clegg, E.D., Selevan, S.G. & Brown, C.C. Estimates of human fertility and pregnancy loss. Fertil. Steril. 65, 503–509 (1996).
Clark, D.A. Immunological factors in pregnancy wastage: fact or fiction. Am. J. Reprod. Immunol. 59, 277–300 (2008).
Redman, C.W. & Sargent, I.L. Latest advances in understanding preeclampsia. Science 308, 1592–1594 (2005).
Curotto de Lafaille, M.A. & Lafaille, J.J. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity 30, 626–635 (2009).
Andersen, K.G., Nissen, J.K. & Betz, A.G. Comparative Genomics Reveals Key Gain-of-Function Events in Foxp3 during Regulatory T Cell Evolution. Front. Immunol. 3, 113 (2012).
Samstein, R.M., Josefowicz, S.Z., Arvey, A., Treuting, P.M. & Rudensky, A.Y. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell 150, 29–38 (2012).
Yadav, M. et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J. Exp. Med. 209, 1713–1722 (2012).
Blois, S.M. et al. Depletion of CD8+ cells abolishes the pregnancy protective effect of progesterone substitution with dydrogesterone in mice by altering the Th1/Th2 cytokine profile. J. Immunol. 172, 5893–5899 (2004).
Tilburgs, T. et al. Differential distribution of CD4+CD25bright and CD8+CD28− T-cells in decidua and maternal blood during human pregnancy. Placenta 27, S47–S53 (2006).
Norton, M.T., Fortner, K.A., Oppenheimer, K.H. & Bonney, E. Evidence that CD8 T-cell homeostasis and function remain intact during murine pregnancy. Immunology 131, 426–437 (2010).
Rieger, L. et al. Specific subsets of immune cells in human decidua differ between normal pregnancy and preeclampsia—a prospective observational study. Reprod. Biol. Endocrinol. 7, 132 (2009).
Aït-Azzouzene, D. et al. Transgenic major histocompatibility complex class I antigen expressed in mouse trophoblast affects maternal immature B cells. Biol. Reprod. 65, 337–344 (2001).
Medina, K.L., Smithson, G. & Kincade, P.W. Suppression of B lymphopoiesis during normal pregnancy. J. Exp. Med. 178, 1507–1515 (1993).
Shao, L., Jacobs, A.R., Johnson, V.V. & Mayer, L. Activation of CD8+ regulatory T cells by human placental trophoblasts. J. Immunol. 174, 7539–7547 (2005).
Ernest, J.M., Marshburn, P.B. & Kutteh, W.H. Obstetric antiphospholipid syndrome: an update on pathophysiology and management. Semin. Reprod. Med. 29, 522–539 (2011).
Veltman-Verhulst, S.M., Cohlen, B.J., Hughes, E. & Heineman, M.J. Intra-uterine insemination for unexplained subfertility. Cochrane Database Syst. Rev. 9, CD001838 (2012).
Chard, T. Frequency of implantation and early pregnancy loss in natural cycles. Baillieres Clin. Obstet. Gynaecol. 5, 179–189 (1991).
Wilcox, A.J. et al. Incidence of early loss of pregnancy. N. Engl. J. Med. 319, 189–194 (1988).
Rai, R. & Regan, L. Recurrent miscarriage. Lancet 368, 601–611 (2006).
Scherjon, S., Lashley, L., van der Hoorn, M.L. & Claas, F. Fetus specific T cell modulation during fertilization, implantation and pregnancy. Placenta 32 (suppl. 4), S291–S297 (2011).
Clark, D.A., Chaouat, G., Guenet, J.L. & Kiger, N. Local active suppression and successful vaccination against spontaneous abortion in CBA/J mice. J. Reprod. Immunol. 10, 79–85 (1987).
Liu, A.X. et al. Proteomic analysis on the alteration of protein expression in the placental villous tissue of early pregnancy loss. Biol. Reprod. 75, 414–420 (2006).
Jeschke, U., Toth, B., Scholz, C., Friese, K. & Makrigiannakis, A. Glycoprotein and carbohydrate binding protein expression in the placenta in early pregnancy loss. J. Reprod. Immunol. 85, 99–105 (2010).
Jin, L.P., Chen, Q.Y., Zhang, T., Guo, P.F. & Li, D.J. The CD4+CD25bright regulatory T cells and CTLA-4 expression in peripheral and decidual lymphocytes are down-regulated in human miscarriage. Clin. Immunol. 133, 402–410 (2009).
Shimada, S. et al. Natural killer, natural killer T, helper and cytotoxic T cells in the decidua from sporadic miscarriage. Am. J. Reprod. Immunol. 56, 193–200 (2006).
Erlebacher, A., Zhang, D., Parlow, A.F. & Glimcher, L.H. Ovarian insufficiency and early pregnancy loss induced by activation of the innate immune system. J. Clin. Invest. 114, 39–48 (2004).
Mao, G. et al. Progesterone increases systemic and local uterine proportions of CD4+CD25+ Treg cells during midterm pregnancy in mice. Endocrinology 151, 5477–5488 (2010).
Hiby, S.E. et al. Association of maternal killer-cell immunoglobulin-like receptors and parental HLA-C genotypes with recurrent miscarriage. Hum. Reprod. 23, 972–976 (2008).
Gonzalez, J.M., Franzke, C.W., Yang, F., Romero, R. & Girardi, G. Complement activation triggers metalloproteinases release inducing cervical remodeling and preterm birth in mice. Am. J. Pathol. 179, 838–849 (2011).
Haddad, R. et al. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am. J. Obstet. Gynecol. 195, 394.e1–394.24 (2006).
Barker, D.J. Adult consequences of fetal growth restriction. Clin. Obstet. Gynecol. 49, 270–283 (2006).
Gluckman, P.D., Hanson, M.A., Cooper, C. & Thornburg, K.L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 359, 61–73 (2008).
Solano, M.E., Jago, C., Pincus, M.K. & Arck, P.C. Highway to health; or How prenatal factors determine disease risks in the later life of the offspring. J. Reprod. Immunol. 90, 3–8 (2011).
Bach, J.F. The effect of infections on susceptibility to autoimmune and allergic diseases. N. Engl. J. Med. 347, 911–920 (2002).
Mold, J.E. et al. Maternal alloantigens promote the development of tolerogenic fetal regulat. T cells in utero. Science 322, 1562–1565 (2008).
Marleau, A.M. et al. Chimerism of murine fetal bone marrow by maternal cells occurs in late gestation and persists into adulthood. Lab. Invest. 83, 673–681 (2003).
Dutta, P. et al. Microchimerism is strongly correlated with tolerance to noninherited maternal antigens in mice. Blood 114, 3578–3587 (2009).
Wienecke, J. et al. Pro-inflammatory effector Th cells transmigrate through anti-inflammatory environments into the murine fetus. Placenta 33, 39–46 (2012).
Nelson, J.L. et al. Maternal microchimerism in peripheral blood in type 1 diabetes and pancreatic islet beta cell microchimerism. Proc. Natl. Acad. Sci. USA 104, 1637–1642 (2007).
Ye, Y. et al. Maternal microchimerism in muscle biopsies from children with juvenile dermatomyositis. Rheumatology 51, 987–991 (2012).
Yan, Z., Aydelotte, T., Gadi, V.K., Guthrie, K.A. & Nelson, J.L. Acquisition of the rheumatoid arthritis HLA shared epitope through microchimerism. Arthritis Rheum. 63, 640–644 (2011).
Kopcow, H.D. et al. T cell apoptosis at the maternal-fetal interface in early human pregnancy, involvement of galectin-1. Proc. Natl. Acad. Sci. USA 105, 18472–18477 (2008).
Vacca, P. et al. Crosstalk between decidual NK and CD14+ myelomonocytic cells results in induction of Tregs and immunosuppression. Proc. Natl. Acad. Sci. USA 107, 11918–11923 (2010).
Hartwig, I.R., Pincus, M.K., Diemert, A., Hecher, K. & Arck, P.C. Sex-specific effect of first-trimester maternal progesterone on birthweight. Hum. Reprod. 28, 77–86 (2013).
Arck, P.C. et al. Early risk factors for miscarriage: a prospective cohort study in pregnant women. Reprod. Biomed. Online 17, 101–113 (2008).
Cannizzo, E.S., Clement, C.C., Sahu, R., Follo, C. & Santambrogio, L. Oxidative stress, inflamm-aging and immunosenescence. J. Proteomics 74, 2313–2323 (2011).
Glaser, R. & Kiecolt-Glaser, J.K. Stress-induced immune dysfunction: implications for health. Nat. Rev. Immunol. 5, 243–251 (2005).
Karimi, K. & Arck, P.C. Natural Killer cells: keepers of pregnancy in the turnstile of the environment. Brain Behav. Immun. 24, 339–347 (2010).
Van Kerkhove, M.D. et al. WHO Working Group for Risk Factors for Severe H1N1. Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS Med. 8, e1001053 (2011).
Thiele, K., Kessler, T., Arck, P., Erhardt, A. & Tiegs, G. Acetaminophen and pregnancy: short- and long-term consequences for mother and child. J. Reprod. Immunol. 97, 128–139 (2013).
Brannon, P.M. Vitamin D and adverse pregnancy outcomes: beyond bone health and growth. Proc. Nutr. Soc. 71, 205–212 (2012).
Thorsby, E. A short history of HLA. Tissue Antigens 74, 101–116 (2009).
Kovats, S. et al. A class I antigen, HLA-G, expressed in human trophoblasts. Science 248, 220–223 (1990).
Apps, R. et al. Human leucocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology 127, 26–39 (2009).
Lim, H.J. & Wang, H. Uterine disorders and pregnancy complications: insights from mouse models. J. Clin. Invest. 120, 1004–1015 (2010).
Madeja, Z. et al. Paternal MHC expression on mouse trophoblast affects uterine vascularization and fetal growth. Proc. Natl. Acad. Sci. USA 108, 4012–4017 (2011).
Confavreux, C., Hutchinson, M., Hours, M.M., Cortinovis-Tourniaire, P. & Moreau, T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N. Engl. J. Med. 339, 285–291 (1998).
de Man, Y.A., Dolhain, R.J., van de Geijn, F.E., Willemsen, S.P. & Hazes, J.M. Disease activity of rheumatoid arthritis during pregnancy: results from a nationwide prospective study. Arthritis Rheum. 59, 1241–1248 (2008).
Vukusic, S. et al. Pregnancy and multiple sclerosis (the PRIMS study): clinical predictors of post-partum relapse. Brain 127, 1353–1360 (2004).
Patas, K., Engler, J.B., Friese, M.A. & Gold, S.M. Pregnancy and multiple sclerosis: fetomaternal immune cross talk and its implications for disease activity. J. Reprod. Immunol. 97, 140–146 (2013).
Munoz-Suano, A., Kallikourdis, M., Sarris, M. & Betz, A.G. Regulatory T cells protect from autoimmune arthritis during pregnancy. J. Autoimmun. 38, J103–J108 (2012).
Ponsonby, A.L. et al. Offspring number, pregnancy, and risk of a first clinical demyelinating event: the AusImmune Study. Neurology 78, 867–874 (2012).
Smyth, A. et al. A systematic review and meta-analysis of pregnancy outcomes in patients with systemic lupus erythematosus and lupus nephritis. Clin. J. Am. Soc. Nephrol. 5, 2060–2068 (2010).
Kuroki, M. et al. Detection of maternal-fetal microchimerism in the inflammatory lesions of patients with Sjogren's syndrome. Ann. Rheum. Dis. 61, 1041–1046 (2002).
Artlett, C.M., Smith, J.B. & Jimenez, S.A. Identification of fetal DNA and cells in skin lesions from women with systemic sclerosis. N. Engl. J. Med. 338, 1186–1191 (1998).
Klintschar, M., Schwaiger, P., Mannweiler, S., Regauer, S. & Kleiber, M. Evidence of fetal microchimerism in Hashimoto's thyroiditis. J. Clin. Endocrinol. Metab. 86, 2494–2498 (2001).
Lateef, A. & Petri, M. Management of pregnancy in systemic lupus erythematosus. Nat. Rev. Rheumatol. 8, 710–718 (2012).
Bloch, E.M. et al. Male microchimerism in peripheral blood leukocytes from women with multiple sclerosis. Chimerism 2, 6–10 (2011).
Chan, W.F. et al. Microchimerism in the rheumatoid nodules of patients with rheumatoid arthritis. Arthritis Rheum. 64, 380–388 (2012).
Acknowledgements
The writing of this review article was made possible by grants provided by the German Research Foundation (AR232/19), the Foundation for Research and Science Hamburg (1232/102), the Werner Otto Foundation (4/79), AllerGen NCE (09B6) and the World Health Organization (A65097) to P.C.A. and K.H.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Arck, P., Hecher, K. Fetomaternal immune cross-talk and its consequences for maternal and offspring's health. Nat Med 19, 548–556 (2013). https://doi.org/10.1038/nm.3160
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3160