Abstract
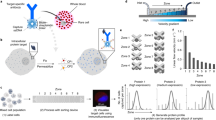
Glioblastomas shed large quantities of small, membrane-bound microvesicles into the circulation. Although these hold promise as potential biomarkers of therapeutic response, their identification and quantification remain challenging. Here, we describe a highly sensitive and rapid analytical technique for profiling circulating microvesicles directly from blood samples of patients with glioblastoma. Microvesicles, introduced onto a dedicated microfluidic chip, are labeled with target-specific magnetic nanoparticles and detected by a miniaturized nuclear magnetic resonance system. Compared with current methods, this integrated system has a much higher detection sensitivity and can differentiate glioblastoma multiforme (GBM) microvesicles from nontumor host cell–derived microvesicles. We also show that circulating GBM microvesicles can be used to analyze primary tumor mutations and as a predictive metric of treatment-induced changes. This platform could provide both an early indicator of drug efficacy and a potential molecular stratifier for human clinical trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Maheswaran, S. et al. Detection of mutations in EGFR in circulating lung-cancer cells. N. Engl. J. Med. 359, 366–377 (2008).
Kulasingam, V., Pavlou, M.P. & Diamandis, E.P. Integrating high-throughput technologies in the quest for effective biomarkers for ovarian cancer. Nat. Rev. Cancer 10, 371–378 (2010).
Simpson, R.J., Lim, J.W., Moritz, R.L. & Mathivanan, S. Exosomes: proteomic insights and diagnostic potential. Expert Rev. Proteomics 6, 267–283 (2009).
Théry, C., Ostrowski, M. & Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 9, 581–593 (2009).
Skog, J. et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 10, 1470–1476 (2008).
Al-Nedawi, K. et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 10, 619–624 (2008).
Simons, M. & Raposo, G. Exosomes—vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 21, 575–581 (2009).
Lee, T.H. et al. Microvesicles as mediators of intercellular communication in cancer—the emerging science of cellular 'debris'. Semin. Immunopathol. 33, 455–467 (2011).
Cocucci, E., Racchetti, G. & Meldolesi, J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 19, 43–51 (2009).
Graner, M.W. et al. Proteomic and immunologic analyses of brain tumor exosomes. FASEB J. 23, 1541–1557 (2009).
Sanderson, M.P. et al. Generation of novel, secreted epidermal growth factor receptor (EGFR/ErbB1) isoforms via metalloprotease-dependent ectodomain shedding and exosome secretion. J. Cell. Biochem. 103, 1783–1797 (2008).
Balaj, L. et al. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun. 2, 180 (2011).
Lee, H., Sun, E., Ham, D. & Weissleder, R. Chip-NMR biosensor for detection and molecular analysis of cells. Nat. Med. 14, 869–874 (2008).
Issadore, D. et al. Miniature magnetic resonance system for point-of-care diagnostics. Lab Chip 11, 2282–2287 (2011).
Haun, J.B. et al. Micro-NMR for rapid molecular analysis of human tumor samples. Sci. Transl. Med. 3, 71ra16 (2011).
Théry, C., Zitvogel, L. & Amigorena, S. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2, 569–579 (2002).
Haun, J.B., Devaraj, N.K., Hilderbrand, S.A., Lee, H. & Weissleder, R. Bioorthogonal chemistry amplifies nanoparticle binding and enhances the sensitivity of cell detection. Nat. Nanotechnol. 5, 660–665 (2010).
Thery, C., Amigorena, S., Raposo, G. & Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 30, 3.22 (2006).
Fleming, T.P. et al. Amplification and/or overexpression of platelet-derived growth factor receptors and epidermal growth factor receptor in human glial tumors. Cancer Res. 52, 4550–4553 (1992).
Mishima, K. et al. Increased expression of podoplanin in malignant astrocytic tumors as a novel molecular marker of malignant progression. Acta Neuropathol. 111, 483–488 (2006).
Wykosky, J., Gibo, D.M., Stanton, C. & Debinski, W. EphA2 as a novel molecular marker and target in glioblastoma multiforme. Mol. Cancer Res. 3, 541–551 (2005).
Parsons, D.W. et al. An integrated genomic analysis of human glioblastoma multiforme. Science 321, 1807–1812 (2008).
Dang, L. et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462, 739–744 (2009).
Italiano, J.E.J., Mairuhu, A.T. & Flaumenhaft, R. Clinical relevance of microparticles from platelets and megakaryocytes. Curr. Opin. Hematol. 17, 578–584 (2010).
Pedersen, N.M. et al. Expression of epidermal growth factor receptor or ErbB3 facilitates geldanamycin-induced down-regulation of ErbB2. Mol. Cancer Res. 7, 275–284 (2009).
Trepel, J., Mollapour, M., Giaccone, G. & Neckers, L. Targeting the dynamic HSP90 complex in cancer. Nat. Rev. Cancer 10, 537–549 (2010).
Zhu, H. et al. The novel Hsp90 inhibitor NXD30001 induces tumor regression in a genetically engineered mouse model of glioblastoma multiforme. Mol. Cancer Ther. 9, 2618–2626 (2010).
Zhu, H. et al. Oncogenic EGFR signaling cooperates with loss of tumor suppressor gene functions in gliomagenesis. Proc. Natl. Acad. Sci. USA 106, 2712–2716 (2009).
Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455, 1061–1068 (2008).
Verhaak, R.G. et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17, 98–110 (2010).
Pedersen, M.W., Meltorn, M., Damstrup, L. & Poulsen, H.S. The type III epidermal growth factor receptor mutation. Biological significance and potential target for anti-cancer therapy. Ann. Oncol. 12, 745–760 (2001).
Mellinghoff, I.K. et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N. Engl. J. Med. 353, 2012–2024 (2005).
Galbán, C.J. et al. The parametric response map is an imaging biomarker for early cancer treatment outcome. Nat. Med. 15, 572–576 (2009).
Galbán, C.J. et al. Prospective analysis of parametric MRI biomarkers: Identification of early and distinct glioma response patterns not predicted by standard radiographic assessment. Clin. Cancer Res. 17, 4751–4760 (2011).
Tsien, C. et al. Parametric response map as an imaging biomarker to distinguish progression from pseudoprogression in high-grade glioma. J. Clin. Oncol. 28, 2293–2299 (2010).
Elkhaled, A. et al. Magnetic resonance of 2-hydroxyglutarate in IDH1-Mutated low-grade gliomas. Sci. Transl. Med. 4, 116ra5 (2012).
Wei, L.H. et al. Changes in tumor metabolism as readout for mammalian target of rapamycin kinase inhibition by rapamycin in glioblastoma. Clin. Cancer Res. 14, 3416–3426 (2008).
Yoon, T.J., Lee, H., Shao, H. & Weissleder, R. Highly magnetic core-shell nanoparticles with a unique magnetization mechanism. Angew. Chem. Int. Edn Engl. 50, 4663–4666 (2011).
Acknowledgements
We thank T. Reiner (Massachusetts General Hospital (MGH)) for preparing TCO, N. Sergeyev (MGH) for synthesizing MNPs, S. Hilderbrand (MGH) for synthesizing reactive TZ, M. Pittet (MGH) for LNZ308 cells and T. Chan (Memorial Sloan-Kettering Cancer Center) for SkMG3 cells, as well as M. Liong and A. Ghazani for assay assistance, B. Marinelli for μNMR measurements, C. Min for software implementation, M. McKee for transmission electron microscopy, J. Skog for advice on NTA measurements, L. Zhu and S. Sivaraman for technical assistance and Y. Fisher-Jeffes for critical reading of the manuscript. Special thanks to C. Castro, J. Carlson and clinical colleagues for many helpful discussions. This work was supported in part by NIH grants U54CA151884, R01EB010011, R01EB004626, P01CA069246, P50CA86355, U01CA141556, U24CA092782 and R21CA14122; H.S. received a BS-PhD National Science Scholarship awarded by the Singapore Agency for Science, Technology and Research; A.C. received an American Cancer Society Research Scholar Award 117409.
Author information
Authors and Affiliations
Contributions
H.S., R.W. and H.L. designed the study. H.S., J.C., L.B. and H.L. performed the experiments. H.S., J.C., R.W. and H.L. analyzed the data and wrote the manuscript. A.C. generated the mouse T103 model. D.D.B. recommended GBM biomarkers and generated the EGFRvIII-specific antibody. B.S.C., F.H.H. and X.O.B. coordinated the clinical study and analyzed the results.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Methods, Supplementary Figures 1–8 and Supplementary Tables 1–4 (PDF 2579 kb)
Rights and permissions
About this article
Cite this article
Shao, H., Chung, J., Balaj, L. et al. Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nat Med 18, 1835–1840 (2012). https://doi.org/10.1038/nm.2994
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2994
This article is cited by
-
Protein cargo in extracellular vesicles as the key mediator in the progression of cancer
Cell Communication and Signaling (2024)
-
Extracellular vesicle-associated tyrosine kinase-like orphan receptors ROR1 and ROR2 promote breast cancer progression
Cell Communication and Signaling (2023)
-
Microfluidic magnetic detection system combined with a DNA framework-mediated immune-sandwich assay for rapid and sensitive detection of tumor-derived exosomes
Microsystems & Nanoengineering (2023)
-
Modeling and optimization of parallelized immunomagnetic nanopore sorting for surface marker specific isolation of extracellular vesicles from complex media
Scientific Reports (2023)
-
Comprehensive insight into endothelial progenitor cell-derived extracellular vesicles as a promising candidate for disease treatment
Stem Cell Research & Therapy (2022)