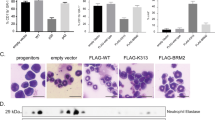
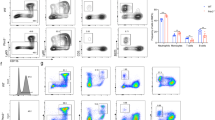
Abstract
We found that hematopoietic cell–specific Lyn substrate 1 (HCLS1 or HS1) is highly expressed in human myeloid cells and that stimulation with granulocyte colony-stimulating factor (G-CSF) leads to HCLS1 phosphorylation. HCLS1 binds the transcription factor lymphoid-enhancer binding factor 1 (LEF-1), transporting LEF-1 into the nucleus upon G-CSF stimulation and inducing LEF-1 autoregulation. In patients with severe congenital neutropenia, inherited mutations in the gene encoding HCLS1-associated protein X-1 (HAX1) lead to profound defects in G-CSF–triggered phosphorylation of HCLS1 and subsequently to reduced autoregulation and expression of LEF-1. Consistent with these results, HCLS1-deficient mice are neutropenic. In bone marrow biopsies of the majority of tested patients with acute myeloid leukemia, HCLS1 protein expression is substantially elevated, associated with high levels of G-CSF synthesis and, in some individuals, a four-residue insertion in a proline-rich region of HCLS1 protein known to accelerate intracellular signaling. These data demonstrate the importance of HCLS1 in myelopoiesis in vitro and in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Kitamura, D., Kaneko, H., Miyagoe, Y., Ariyasu, T. & Watanabe, T. Isolation and characterization of a novel human gene expressed specifically in the cells of hematopoietic lineage. Nucleic Acids Res. 17, 9367–9379 (1989).
van Rossum, A.G., Schuuring-Scholtes, E., van Buuren-van Seggelen, V., Kluin, P.M. & Schuuring, E. Comparative genome analysis of cortactin and HS1: the significance of the F-actin binding repeat domain. BMC Genomics 6, 15 (2005).
Huang, Y. & Burkhardt, J.K. T-cell-receptor–dependent actin regulatory mechanisms. J. Cell Sci. Review 120, 723–730 (2007).
Hao, J.J., Carey, G.B. & Zhan, X. Syk-mediated tyrosine phosphorylation is required for the association of hematopoietic lineage cell-specific protein 1 with lipid rafts and B cell antigen receptor signalosome complex. J. Biol. Chem. 279, 33413–33420 (2004).
Takemoto, Y. et al. Growth factor receptor-bound protein 2 (Grb2) association with hemopoietic specific protein 1: linkage between Lck and Grb2. J. Immunol. 161, 625–630 (1998).
Ingley, E. et al. HS1 interacts with Lyn and is critical for erythropoietin-induced differentiation of erythroid cells. J. Biol. Chem. 275, 7887–7893 (2000).
Yamanashi, Y. et al. Identification of HS1 protein as a major substrate of protein-tyrosine kinase(s) upon B-cell antigen receptor–mediated signaling. Proc. Natl. Acad. Sci. USA 90, 3631–3635 (1993).
Brunati, A.M. et al. Thrombin-induced tyrosine phosphorylation of HS1 in human platelets is sequentially catalyzed by Syk and Lyn tyrosine kinases and associated with the cellular migration of the protein. J. Biol. Chem. 280, 21029–21035 (2005).
Kahner, B.N. et al. Hematopoietic lineage cellspecific protein 1 (HS1) is a functionally important signaling molecule in platelet activation. Blood. 110, 2449–2456 (2007).
Yamanashi, Y. et al. Role of tyrosine phosphorylation of HS1 in B cell antigen receptor-mediated apoptosis. J. Exp. Med. 185, 1387–1392 (1997).
Uruno, T., Zhang, P., Liu, J., Hao, J.J. & Zhan, X. Haematopoietic lineage cell–specific protein 1 (HS1) promotes actin-related protein (Arp) 2/3 complex-mediated actin polymerization. Biochem. J. 371, 485–493 (2003).
Huang, Y. et al. The c-Abl tyrosine kinase regulates actin remodeling at the immune synapse. Blood 112, 111–119 (2008).
Scielzo, C. et al. HS1 protein is differentially expressed in chronic lymphocytic leukemia patient subsets with good or poor prognoses. J. Clin. Invest. 115, 1644–1650 (2005).
Taniuchi, I. et al. Antigen-receptor induced clonal expansion and deletion of lymphocytes are impaired in mice lacking HS1 protein, a substrate of the antigen-receptor-coupled tyrosine kinases. EMBO J. 14, 3664–3678 (1995).
Corey, S.J. et al. Requirement of Src kinase Lyn for induction of DNA synthesis by granulocyte colony-stimulating factor. J. Biol. Chem. 273, 3230–3235 (1998).
Corey, S.J. & Anderson, S.M. Src-related protein tyrosine kinases in hematopoiesis. Blood Review 93, 1–14 (1999).
Ward, A.C., Monkhouse, J.L., Hamilton, J.A. & Csar, X.F. Direct binding of Shc, Grb2, SHP-2 and p40 to the murine granulocyte colony-stimulating factor receptor. Biochim. Biophys. Acta 1448, 70–76 (1998).
Ward, A.C. et al. Tyrosine-dependent and -independent mechanisms of STAT3 activation by the human granulocyte colony-stimulating factor (G-CSF) receptor are differentially utilized depending on G-CSF concentration. Blood 93, 113–124 (1999).
Zhu, Q.S., Robinson, L.J., Roginskaya, V. & Corey, S.J. G-CSF-induced tyrosine phosphorylation of Gab2 is Lyn kinase dependent and associated with enhanced Akt and differentiative, not proliferative, responses. Blood 103, 3305–3312 (2004).
Zhu, Q.S. et al. G-CSF induced reactive oxygen species involves Lyn-PI3-kinase-Akt and contributes to myeloid cell growth. Blood 107, 1847–1856 (2006).
Kendrick, T.S. & Bogoyevitch, M.A. Activation of mitogen-activated protein kinase pathways by the granulocyte colony-stimulating factor receptor: mechanisms and functional consequences. Front. Biosci. Review 12, 591–607 (2007).
Skokowa, J. et al. NAMPT is essential for the G-CSF–induced myeloid differentiation via a NAD+–sirtuin-1–dependent pathway. Nat. Med. 15, 151–158 (2009).
Akbarzadeh, S. et al. Tyrosine residues of the granulocyte colony-stimulating factor receptor transmit proliferation and differentiation signals in murine bone marrow cells. Blood 99, 879–887 (2002).
Corey, S.J. et al. Granulocyte colony–stimulating factor receptor signaling involves the formation of a three-component complex with Lyn and Syk protein-tyrosine kinases. Proc. Natl. Acad. Sci. USA 91, 4683–4687 (1994).
Ward, A.C. et al. The SH2 domain–containing protein tyrosine phosphatase SHP-1 is induced by granulocyte colony-stimulating factor (G-CSF) and modulates signaling from the G-CSF receptor. Leukemia 14, 1284–1291 (2000).
Basu, S., Dunn, A. & Ward, A. G-CSF function and modes of action. Int. J. Mol. Med. 10, 3–10 (2002).
Welte, K., Zeidler, C. & Dale, D.C. Severe congenital neutropenia. Semin. Hematol. 43, 189–195 (2006).
Zeidler, C., Germeshausen, M., Klein, C. & Welte, K. Clinical implications of ELA2-, HAX1-, and G-CSF-receptor (CSF3R) mutations in severe congenital neutropenia. Br. J. Haematol. 144, 459–467 (2009).
Klein, C. et al. HAX1 deficiency causes autosomal recessive severe congenital neutropenia (Kostmann disease). Nat. Genet. 39, 86–92 (2007).
Suzuki, Y. et al. HAX-1, a novel intracellular protein, localized on mitochondria, directly associates with HS1, a substrate of Src family tyrosine kinases. J. Immunol. 158, 2736–2744 (1997).
Chao, J.R. et al. Hax1-mediated processing of HtrA2 by Parl allows survival of lymphocytes and neurons. Nature 452, 98–102 (2008).
Vafiadaki, E. et al. The anti-apoptotic protein HAX-1 interacts with SERCA2 and regulates its protein levels to promote cell survival. Mol. Biol. Cell 20, 306–318 (2009).
Al-Maghrebi, M. et al. The 3′ untranslated region of human vimentin mRNA interacts with protein complexes containing eEF-1γ and HAX-1. Nucleic Acids Res. 30, 5017–5028 (2002).
Skokowa, J. et al. LEF-1 is crucial for neutrophil granulocytopoiesis and its expression is severely reduced in congenital neutropenia. Nat. Med. 12, 1191–1197 (2006).
Elsner, J., Roesler, J., Emmendörffer, A., Lohmann-Matthes, M.L. & Welte, K. Abnormal regulation in the signal transduction in neutrophils from patients with severe congenital neutropenia: relation of impaired mobilization of cytosolic free calcium to altered chemotaxis, superoxide anion generation and F-actin content. Exp. Hematol. 21, 38–46 (1993).
Eastman, Q. & Grosschedl, R. Regulation of LEF-1/TCF transcription factors by Wnt and other signals. Curr. Opin. Cell Biol. Review 11, 233–240 (1999).
Timm, A. & Grosschedl, R. Wnt signaling in lymphopoiesis. Curr. Top. Microbiol. Immunol. Review 290, 225–252 (2005).
Bruhn, L., Munnerlyn, A. & Grosschedl, R. ALY, a context-dependent coactivator of LEF-1 and AML-1, is required for TCRα enhancer function. Genes Dev. 11, 640–653 (1997).
Vadlamudi, U. et al. PITX2, β-catenin and LEF-1 interact to synergistically regulate the LEF-1 promoter. J. Cell Sci. 118, 1129–1137 (2005).
Levanon, D. et al. Transcriptional repression by AML1 and LEF-1 is mediated by the TLE/Groucho corepressors. Proc. Natl. Acad. Sci. USA 95, 11590–11595 (1998).
Petropoulos, K. et al. A novel role for Lef-1, a central transcription mediator of Wnt signaling, in leukemogenesis. J. Exp. Med. 205, 515–522 (2008).
Yaffe, M.B. et al. A motif-based profile scanning approach for genome-wide prediction of signaling pathways. Nat. Biotechnol. 19, 348–353 (2001).
Songyang, Z. et al. Use of an oriented peptide library to determine the optimal substrates of protein kinases. Curr. Biol. 4, 973–982 (1994).
Söderberg, O. et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat. Methods 3, 995–1000 (2006).
Nilsson, I. et al. VEGF receptor 2/-3 heterodimers detected in situ by proximity ligation on angiogenic sprouts. EMBO J. 29, 1377–1388 (2010).
Greenberg, J.I. et al. A role for VEGF as a negative regulator of pericyte function and vessel maturation. Nature 456, 809–813 (2008).
Elsner, J., Roesler, J., Emmendörffer, A., Lohmann-Matthes, M.L. & Welte, K. Abnormal regulation in the signal transduction in neutrophils from patients with severe congenital neutropenia: relation of impaired mobilization of cytosolic free calcium to altered chemotaxis, superoxide anion generation and F-actin content. Exp. Hematol. 21, 38–46 (1993).
Otsuka, J. et al. Association of a four-amino acid residue insertion polymorphism of the HS1 gene with systemic lupus erythematosus: molecular and functional analysis. Arthritis Rheum. 50, 871–881 (2004).
Prieve, M.G., Guttridge, K.L., Munguia, J. & Waterman, M.L. Differential importin-α recognition and nuclear transport by nuclear localization signals within the high-mobility-group DNA binding domains of lymphoid enhancer factor 1 and T-cell factor 1. Mol. Cell. Biol. 18, 4819–4832 (1998).
Prieve, M.G., Guttridge, K.L., Munguia, J.E. & Waterman, M.L. The nuclear localization signal of lymphoid enhancer factor-1 is recognized by two differentially expressed Srp1-nuclear localization sequence receptor. J. Biol. Chem. 271, 7654–7658 (1996).
Asally, M. & Yoneda, Y. β-catenin can act as a nuclear import receptor for its partner transcription factor, lymphocyte enhancer factor-1 (lef-1). Exp. Cell Res. 308, 357–363 (2005).
Coyle-Rink, J., Del Valle, L., Sweet, T., Khalili, K. & Amini, S. Developmental expression of Wnt signaling factors in mouse brain. Cancer Biol. Ther. 1, 640–645 (2002).
Arsenian, S., Weinhold, B., Oelgeschläger, M., Rüther, U. & Nordheim, A. Serum response factor is essential for mesoderm formation during mouse embryogenesis. EMBO J. 17, 6289–6299 (1998).
Luderer, H.F., Gori, F. & Demay, M.B. Lymphoid enhancer-binding factor-1 (LEF1) interacts with the DNA-binding domain of the vitamin D receptor. J. Biol. Chem. 286, 18444–18451 (2011).
Colaluca, I.N. et al. NUMB controls p53 tumour suppressor activity. Nature 451, 76–80 (2008).
Fonseca, A.V., Freund, D., Bornhäuser, M. & Corbeil, D. Polarization and migration of hematopoietic stem and progenitor cells rely on the RhoA/ROCK I pathway and an active reorganization of the microtubule network. J. Biol. Chem. 285, 31661–31671 (2010).
Hagiwara, S. et al. Tyrosine phosphorylation of proteins in primary human myeloid leukemia cells stimulated by cytokines: analysis of the frequency of phosphorylation, and partial identification and semi-quantification of signaling molecules. Int. J. Hematol. 68, 387–401 (1998).
Young, D.C., Wagner, K. & Griffin, J.D. Constitutive expression of the granulocyte-macrophage colony–stimulating factor gene in acute myeloblastic leukemia. J. Clin. Invest. 79, 100–106 (1987).
Young, D.C., Demetri, G.D., Ernst, T.J., Cannistra, S.A. & Griffin, J.D. In vitro expression of colony-stimulating factor genes by human acute myeloblastic leukemia cells. Exp. Hematol. 16, 378–382 (1988).
Ernst, T.J., Ritchie, A.R., O'Rourke, R. & Griffin, J.D. Colony-stimulating factor gene expression in human acute myeloblastic leukemia cells is posttranscriptionally regulated. Leukemia 3, 620–625 (1989).
Acknowledgements
We thank A. Gigina, K. Cherkaoui, A. Müller Brechlin, M. Reuter and A.-L. Hagemann for technical assistance. We thank J. Klupp for assistance in generation of LEF-1 Ala16 mutant; D.D. Billadeau (Department of Immunology, Schulze Center for Novel Therapeutics, College of Medicine, Mayo Clinic) for providing us with the rabbit polyclonal HCLS1-specific antibody; L. Naldini (San Raffaele Telethon Institute for Gene Therapy, Division of Regenerative Medicine, Gene Therapy and Stem Cells, San Raffaele Institute) for pRRL.PPT.PGK.GFPpre vector; A. Schambach (Department of Experimental Hematology, Hannover Medical School) for VSVg envelope plasmid; Thomas J. Hope (University of Illinois at Chicago) for pRSV-Rev plasmid; and O. Tetsu (Department of Otolaryngology—Head and Neck Surgery, University of California–San Francisco) for the CCND1 reporter construct. We also thank the physicians within the Severe Chronic Neutropenia International Registry for providing patient material. We thank study subjects for their cooperation. This work was partially supported by German José Carreras Leukemia Foundation (J.S., M.K.), by REBIRTH Cluster of Excellence of the Hannover Medical School (J.S.), by Madeleine Schickedanz-KinderKrebs-Stiftung (J.S.), and by the Deutsche Forschungsgemeinschaft (Z.L.; DFG grant Li 1608/2-1).
Author information
Authors and Affiliations
Contributions
K.W. and J.S. made initial observations, designed the experiments, analyzed the data, supervised experimentation and wrote the manuscript; J.S., D.L., M.K., O.K., A.G. and I.K. performed the main experiments; K.G. introduced mutations into LEF-1 and C/EBPα reporter gene constructs and performed reporter gene assays in HEK293T cells; J.B. and E.C. performed blood cell counting in Hcls1−/− mice and provided bone marrow and blood material of these mice; K.H. and H.-H.K. performed tissue microarray analysis; Z.L. and A.G. provided some of the AML samples; C.S. and R.G. introduced mutations in LEF-1 cDNA corresponding to HCLS1-binding site; C.Z. provided patient material.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–18, Supplementary Tables 2–5 and Supplementary Methods (PDF 2905 kb)
Supplementary Table 1
Microarray data of CD34+ cells transduced with HCLS1 shRNA vs ctrl shRNA (XLS 6153 kb)
Rights and permissions
About this article
Cite this article
Skokowa, J., Klimiankou, M., Klimenkova, O. et al. Interactions among HCLS1, HAX1 and LEF-1 proteins are essential for G-CSF–triggered granulopoiesis. Nat Med 18, 1550–1559 (2012). https://doi.org/10.1038/nm.2958
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2958