Abstract
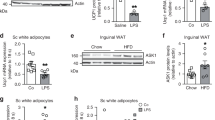
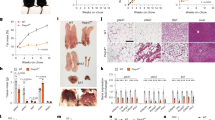
Promoting brown adipose tissue (BAT) formation and function may reduce obesity. Recent data link retinoids to energy balance, but a specific role for retinoid metabolism in white versus brown fat is unknown. Retinaldehyde dehydrogenases (Aldhs), also known as aldehyde dehydrogenases, are rate-limiting enzymes that convert retinaldehyde (Rald) to retinoic acid. Here we show that Aldh1a1 is expressed predominately in white adipose tissue (WAT), including visceral depots in mice and humans. Deficiency of the Aldh1a1 gene induced a BAT-like transcriptional program in WAT that drove uncoupled respiration and adaptive thermogenesis. WAT-selective Aldh1a1 knockdown conferred this BAT program in obese mice, limiting weight gain and improving glucose homeostasis. Rald induced uncoupling protein-1 (Ucp1) mRNA and protein levels in white adipocytes by selectively activating the retinoic acid receptor (RAR), recruiting the coactivator PGC-1α and inducing Ucp1 promoter activity. These data establish Aldh1a1 and its substrate Rald as previously unrecognized determinants of adipocyte plasticity and adaptive thermogenesis, which may have potential therapeutic implications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Mensah, G.A. et al. Obesity, metabolic syndrome, and type 2 diabetes: emerging epidemics and their cardiovascular implications. Cardiol. Clin. 22, 485–504 (2004).
Bray, G.A. & Bellanger, T. Epidemiology, trends, and morbidities of obesity and the metabolic syndrome. Endocrine 29, 109–117 (2006).
Klein, S. et al. Waist circumference and cardiometabolic risk: a consensus statement from shaping America's health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Diabetes Care 30, 1647–1652 (2007).
Cannon, B. & Nedergaard, J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359 (2004).
Farmer, S.R. Molecular determinants of brown adipocyte formation and function. Genes Dev. 22, 1269–1275 (2008).
Seale, P., Kajimura, S. & Spiegelman, B.M. Transcriptional control of brown adipocyte development and physiological function—of mice and men. Genes Dev. 23, 788–797 (2009).
Cypess, A.M. et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360, 1509–1517 (2009).
Tseng, Y.H. et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 454, 1000–1004 (2008).
Seale, P. et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 454, 961–967 (2008).
Vegiopoulos, A. et al. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science 328, 1158–1161 (2010).
Hansen, J.B. et al. Retinoblastoma protein functions as a molecular switch determining white versus brown adipocyte differentiation. Proc. Natl. Acad. Sci. USA 101, 4112–4117 (2004).
Fang, S. et al. Corepressor SMRT promotes oxidative phosphorylation in adipose tissue and protects against diet-induced obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 108, 3412–3417 (2011).
Cinti, S. Between brown and white: novel aspects of adipocyte differentiation. Ann. Med. 43, 104–115 (2011).
Park, K.W., Halperin, D.S. & Tontonoz, P. Before they were fat: adipocyte progenitors. Cell Metab. 8, 454–457 (2008).
Schwarz, E.J., Reginato, M.J., Shao, D., Krakow, S.L. & Lazar, M.A. Retinoic acid blocks adipogenesis by inhibiting C/EBPβ-mediated transcription. Mol. Cell. Biol. 17, 1552–1561 (1997).
Ziouzenkova, O. et al. Retinaldehyde represses adipogenesis and diet-induced obesity. Nat. Med. 13, 695–702 (2007).
Kane, M.A. et al. CrbpI modulates glucose homeostasis and pancreas 9-cis-retinoic acid concentrations. Mol. Cell. Biol. 31, 3277–3285 (2011).
Altucci, L., Leibowitz, M.D., Ogilvie, K.M., de Lera, A.R. & Gronemeyer, H. RAR and RXR modulation in cancer and metabolic disease. Nat. Rev. Drug Discov. 6, 793–810 (2007).
Villarroya, F., Iglesias, R. & Giralt, M. Retinoids and retinoid receptors in the control of energy balance: novel pharmacological strategies in obesity and diabetes. Curr. Med. Chem. 11, 795–805 (2004).
Ross, A.C. Overview of retinoid metabolism. J. Nutr. 123, 346–350 (1993).
Ziouzenkova, O. & Plutzky, J. Retinoid metabolism and nuclear receptor responses: New insights into coordinated regulation of the PPAR-RXR complex. FEBS Lett. 582, 32–38 (2008).
Duester, G., Mic, F.A. & Molotkov, A. Cytosolic retinoid dehydrogenases govern ubiquitous metabolism of retinol to retinaldehyde followed by tissue-specific metabolism to retinoic acid. Chem. Biol. Interact. 143–144, 201–210 (2003).
Molotkov, A. & Duester, G. Genetic evidence that retinaldehyde dehydrogenase Raldh1 (Aldh1a1) functions downstream of alcohol dehydrogenase Adh1 in metabolism of retinol to retinoic acid. J. Biol. Chem. 278, 36085–36090 (2003).
Wiegand, G. & Remington, S.J. Citrate synthase: structure, control, and mechanism. Annu. Rev. Biophys. Biophys. Chem. 15, 97–117 (1986).
Trounce, I.A., Kim, Y.L., Jun, A.S. & Wallace, D.C. Assessment of mitochondrial oxidative phosphorylation in patient muscle biopsies, lymphoblasts, and transmitochondrial cell lines. Methods Enzymol. 264, 484–509 (1996).
Tang, Q.Q., Otto, T.C. & Lane, M.D. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc. Natl. Acad. Sci. USA 101, 9607–9611 (2004).
Chute, J.P. et al. Inhibition of aldehyde dehydrogenase and retinoid signaling induces the expansion of human hematopoietic stem cells. Proc. Natl. Acad. Sci. USA 103, 11707–11712 (2006).
Johnson, A.T., Wang, L., Gillett, S.J. & Chandraratna, R.A. High affinity retinoic acid receptor antagonists: analogs of AGN 193109. Bioorg. Med. Chem. Lett. 9, 573–576 (1999).
Takahashi, B. et al. Novel retinoid X receptor antagonists: specific inhibition of retinoid synergism in RXR-RAR heterodimer actions. J. Med. Chem. 45, 3327–3330 (2002).
Puigserver, P. et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92, 829–839 (1998).
Alvarez, R. et al. A novel regulatory pathway of brown fat thermogenesis. Retinoic acid is a transcriptional activator of the mitochondrial uncoupling protein gene. J. Biol. Chem. 270, 5666–5673 (1995).
Cypess, A.M. & Kahn, C.R. Brown fat as a therapy for obesity and diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 17, 143–149 (2010).
Langin, D. Recruitment of brown fat and conversion of white into brown adipocytes: strategies to fight the metabolic complications of obesity? Biochim. Biophys. Acta 1801, 372–376 (2010).
Guerra, C., Koza, R.A., Yamashita, H., Walsh, K. & Kozak, L.P. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J. Clin. Invest. 102, 412–420 (1998).
Seale, P. et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J. Clin. Invest. 121, 96–105 (2011).
Silva, J.E. & Rabelo, R. Regulation of the uncoupling protein gene expression. Eur. J. Endocrinol. 136, 251–264 (1997).
Repa, J.J., Hanson, K.K. & Clagett-Dame, M. All-trans-retinol is a ligand for the retinoic acid receptors. Proc. Natl. Acad. Sci. USA 90, 7293–7297 (1993).
Schug, T.T., Berry, D.C., Shaw, N.S., Travis, S.N. & Noy, N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell 129, 723–733 (2007).
Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Yang, Q. et al. Nature 436, 356–362 (2005).
Crooke, S.T. Progress in antisense technology. Annu. Rev. Med. 55, 61–95 (2004).
Baker, B.F. et al. 2′-O-(2-methoxy)ethyl-modified anti-intercellular adhesion molecule 1 (ICAM-1) oligonucleotides selectively increase the ICAM-1 mRNA level and inhibit formation of the ICAM-1 translation initiation complex in human umbilical vein endothelial cells. J. Biol. Chem. 272, 11994–12000 (1997).
Wernstedt, I. et al. Reduced stress- and cold-induced increase in energy expenditure in interleukin-6-deficient mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R551–R557 (2006).
Hodges, M.R. et al. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J. Neurosci. 28, 2495–2505 (2008).
Kennedy, A.R. et al. A high-fat, ketogenic diet induces a unique metabolic state in mice. Am. J. Physiol. Endocrinol. Metab. 292, E1724–E1739 (2007).
Xu, J. Preparation, culture and immortalization of mouse embryonic fibroblasts. Curr. Protoc. Mol. Biol. 70, 28.1.1–28.1.8 (2005).
Brown, J.D., Oligino, E., Rader, D.J., Saghatelian, A. & Plutzky, J. VLDL hydrolysis by hepatic lipase regulates PPARδ transcriptional responses. PLoS ONE 6, e21209 (2011).
Yehuda-Shnaidman, E., Buehrer, B., Pi, J., Kumar, N. & Collins, S. Acute stimulation of white adipocyte respiration by PKA-induced lipolysis. Diabetes 59, 2474–2483 (2010).
Wu, M. et al. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am. J. Physiol. Cell Physiol. 292, C125–C136 (2007).
Acknowledgements
We thank H. Wang, G. Sukhova, E. Shvartz, T.A. Dang and V. Demchev for excellent technical support, R. Koza (Pennington Biomedical Research Center) for providing the Ucp1 promoter luciferase construct, H. Kagechika (University of Tokyo) for providing HX531 and G. Duester (Burnham Medical Research Institute) for the Aldh1a1−/− mice and helpful discussions. This work was supported by the US National Institutes of Health grants HL048743, AR054604-03S1, 5P30DK057521-12 (J.P.); Mary K. Iacocca Professorship DK082659 and the National Institute of Diabetes and Digestive and Kidney Diseases DK056626 (C.R.K.); DK048873 and DK048873-14S2 (D.E.C.); the Austrian Science Fund (FWF); J3107-B19 (F.W.K.).
Author information
Authors and Affiliations
Contributions
F.W.K. and J.P. designed the study; F.W.K., C.V., P.O., S.S., J.D.B., S.N. and M.Z. researched data; F.W.K. wrote the manuscript; C.V., J.D.B., S.N., M.Z., T.M.S., D.E.C., C.R.K. and J.P. reviewed the manuscript; D.E.C. and C.R.K. contributed to discussion.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 (PDF 3136 kb)
Rights and permissions
About this article
Cite this article
Kiefer, F., Vernochet, C., O'Brien, P. et al. Retinaldehyde dehydrogenase 1 regulates a thermogenic program in white adipose tissue. Nat Med 18, 918–925 (2012). https://doi.org/10.1038/nm.2757
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2757
This article is cited by
-
Retinoid homeostasis in major depressive disorder
Translational Psychiatry (2023)
-
Reconnoitring the Usage of Agroindustrial Waste in Carotenoid Production for Food Fortification: a Sustainable Approach to Tackle Vitamin A Deficiency
Food and Bioprocess Technology (2023)
-
All-trans retinoic acid impairs glucose-stimulated insulin secretion by activating the RXR/SREBP-1c/UCP2 pathway
Acta Pharmacologica Sinica (2022)
-
Wt1 haploinsufficiency induces browning of epididymal fat and alleviates metabolic dysfunction in mice on high-fat diet
Diabetologia (2022)
-
CD10 marks non-canonical PPARγ-independent adipocyte maturation and browning potential of adipose-derived stem cells
Stem Cell Research & Therapy (2021)