Abstract
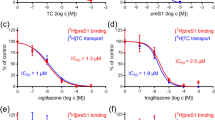
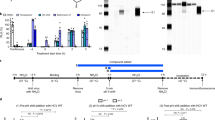
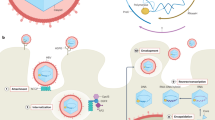
Hepatitis C virus (HCV) is a leading cause of liver disease worldwide. With ∼170 million individuals infected and current interferon-based treatment having toxic side effects and marginal efficacy, more effective antivirals are crucially needed1. Although HCV protease inhibitors were just approved by the US Food and Drug Administration (FDA), optimal HCV therapy, analogous to HIV therapy, will probably require a combination of antivirals targeting multiple aspects of the viral lifecycle. Viral entry represents a potential multifaceted target for antiviral intervention; however, to date, FDA-approved inhibitors of HCV cell entry are unavailable. Here we show that the cellular Niemann-Pick C1–like 1 (NPC1L1) cholesterol uptake receptor is an HCV entry factor amendable to therapeutic intervention. Specifically, NPC1L1 expression is necessary for HCV infection, as silencing or antibody-mediated blocking of NPC1L1 impairs cell culture–derived HCV (HCVcc) infection initiation. In addition, the clinically available FDA-approved NPC1L1 antagonist ezetimibe2,3 potently blocks HCV uptake in vitro via a virion cholesterol–dependent step before virion-cell membrane fusion. Moreover, ezetimibe inhibits infection by all major HCV genotypes in vitro and in vivo delays the establishment of HCV genotype 1b infection in mice with human liver grafts. Thus, we have not only identified NPC1L1 as an HCV cell entry factor but also discovered a new antiviral target and potential therapeutic agent.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Uprichard, S.L. Hepatitis C virus experimental model systems and antiviral drug research. Virol. Sin. 25, 227–245 (2010).
Gupta, E.K. & Ito, M.K. Ezetimibe: the first in a novel class of selective cholesterol-absorption inhibitors. Heart Dis. 4, 399–409 (2002).
Garcia-Calvo, M. et al. The target of ezetimibe is Niemann-Pick C1-like 1 (NPC1L1). Proc. Natl. Acad. Sci. USA 102, 8132–8137 (2005).
Pileri, P. et al. Binding of hepatitis C virus to CD81. Science 282, 938–941 (1998).
Scarselli, E. et al. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J. 21, 5017–5025 (2002).
Evans, M.J. et al. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature 446, 801–805 (2007).
Ploss, A. et al. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature 457, 882–886 (2009).
Liu, S. et al. Tight junction proteins claudin-1 and occludin control hepatitis C virus entry and are downregulated during infection to prevent superinfection. J. Virol. 83, 2011–2014 (2009).
Tscherne, D.M. et al. Time- and temperature-dependent activation of hepatitis C virus for low-pH–triggered entry. J. Virol. 80, 1734–1741 (2006).
Meertens, L., Bertaux, C. & Dragic, T. Hepatitis C virus entry requires a critical postinternalization step and delivery to early endosomes via clathrin-coated vesicles. J. Virol. 80, 11571–11578 (2006).
Huang, H. et al. Hepatitis C virus production by human hepatocytes dependent on assembly and secretion of very low-density lipoproteins. Proc. Natl. Acad. Sci. USA 104, 5848–5853 (2007).
Gastaminza, P. et al. Cellular determinants of hepatitis C virus assembly, maturation, degradation, and secretion. J. Virol. 82, 2120–2129 (2008).
Aizaki, H. et al. Critical role of virion-associated cholesterol and sphingolipid in hepatitis C virus infection. J. Virol. 82, 5715–5724 (2008).
Kapadia, S.B., Barth, H., Baumert, T., McKeating, J.A. & Chisari, F.V. Initiation of hepatitis C virus infection is dependent on cholesterol and cooperativity between CD81 and scavenger receptor B type I. J. Virol. 81, 374–383 (2007).
Yu, L. The structure and function of Niemann-Pick C1–like 1 protein. Curr. Opin. Lipidol. 19, 263–269 (2008).
Altmann, S.W. et al. Niemann-Pick C1 like 1 protein is critical for intestinal cholesterol absorption. Science 303, 1201–1204 (2004).
Sané, A.T. et al. Localization and role of NPC1L1 in cholesterol absorption in human intestine. J. Lipid Res. 47, 2112–2120 (2006).
Bays, H.E., Neff, D., Tomassini, J.E. & Tershakovec, A.M. Ezetimibe: cholesterol lowering and beyond. Expert Rev. Cardiovasc. Ther. 6, 447–470 (2008).
Chang, T.Y. & Chang, C. Ezetimibe blocks internalization of the NPC1L1/cholesterol complex. Cell Metab. 7, 469–471 (2008).
Weinglass, A.B. et al. Extracellular loop C of NPC1L1 is important for binding to ezetimibe. Proc. Natl. Acad. Sci. USA 105, 11140–11145 (2008).
Gottwein, J.M. et al. Development and characterization of hepatitis C virus genotype 1–7 cell culture systems: role of CD81 and scavenger receptor class B type I and effect of antiviral drugs. Hepatology 49, 364–377 (2009).
Grove, J. et al. Scavenger receptor BI and BII expression levels modulate hepatitis C virus infectivity. J. Virol. 81, 3162–3169 (2007).
Zaitseva, E., Yang, S.T., Melikov, K., Pourmal, S. & Chernomordik, L.V. Dengue virus ensures its fusion in late endosomes using compartment-specific lipids. PLoS Pathog. 6, e1001131 (2010).
Coller, K.E. et al. RNA interference and single particle tracking analysis of hepatitis C virus endocytosis. PLoS Pathog. 5, e1000702 (2009).
Zhang, J.H. et al. The N-terminal domain of NPC1L1 protein binds cholesterol and plays essential roles in cholesterol uptake. J. Biol. Chem. 286, 25088–25097 (2011).
Yamamoto, M. et al. Structural requirements of virion-associated cholesterol for infectivity, buoyant density and apolipoprotein association of hepatitis C virus. J. Gen. Virol. 92, 2082–2087 (2011).
Farquhar, M.J. & McKeating, J.A. Primary hepatocytes as targets for hepatitis C virus replication. J. Viral Hepat. 15, 849–854 (2008).
Zhong, J. et al. Persistent hepatitis C virus infection in vitro: coevolution of virus and host. J. Virol. 80, 11082–11093 (2006).
Kneteman, N.M. & Toso, C. In vivo study of HCV in mice with chimeric human livers. Methods Mol. Biol. 510, 383–399 (2009).
Lupberger, J. et al. EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat. Med. 17, 589–595 (2011).
Sweeney, M.E. & Johnson, R.R. Ezetimibe: an update on the mechanism of action, pharmacokinetics and recent clinical trials. Expert Opin. Drug Metab. Toxicol. 3, 441–450 (2007).
Betters, J.L. & Yu, L. Transporters as drug targets: discovery and development of NPC1L1 inhibitors. Clin. Pharmacol. Ther. 87, 117–121 (2010).
Davies, J.P., Scott, C., Oishi, K., Liapis, A. & Ioannou, Y.A. Inactivation of NPC1L1 causes multiple lipid transport defects and protects against diet-induced hypercholesterolemia. J. Biol. Chem. 280, 12710–12720 (2005).
Davis, H.R. Jr. et al. Niemann-Pick C1 like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J. Biol. Chem. 279, 33586–33592 (2004).
Matsumura, T. et al. Amphipathic DNA polymers inhibit hepatitis C virus infection by blocking viral entry. Gastroenterology 137, 673–681 (2009).
Wakita, T. et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med. 11, 791–796 (2005).
Sabahi, A. et al. The rate of hepatitis C virus infection initiation in vitro is directly related to particle density. Virology 407, 110–119 (2010).
Yu, X. & Uprichard, S.L. Cell-based hepatitis C virus infection fluorescence resonance energy transfer (FRET) assay for antiviral compound screening. Curr. Protoc. Microbiol. 18, 17.5.1–17.5.27 (2010).
Ge, L. et al. The cholesterol absorption inhibitor ezetimibe acts by blocking the sterol-induced internalization of NPC1L1. Cell Metab. 7, 508–519 (2008).
Tateno, C. et al. Near completely humanized liver in mice shows human-type metabolic responses to drugs. Am. J. Pathol. 165, 901–912 (2004).
Acknowledgements
We thank T. Wakita (National Institute of Infectious Diseases, Japan) for JFH1-based plasmids, F. Chisari (The Scripps Research Institute) for Huh7 cells, J. Buhk (Copenhagen University Hospital Hepatitis C Program) for JFH-1–based intergenotypic HCV clones, Y. Ioannou (Mount Sinai School of Medicine) for the antibody (Bsn4052) against NPC1L1, D. Burton and M. Law (The Scripps Research Institute) for the antibody (C1) against the HCV glycoprotein E2 and C. Rice (The Rockefeller Institute) for the antibody (E910) against the HCV nonstructural protein NS5A. We would also like to thank P. Corcoran for outstanding technical assistance, H. Dahari for assistance with statistical analyses and T. Layden and S. Cotler for editing the manuscript. We also thank J. Graves of the University of Illinois at Chicago (UIC) Research Resources Center Flow Cytometry laboratory and K. Ma of the UIC Research Resources Center Confocal Microscopy laboratory for technical assistance. This work was supported by the US National Institutes of Health Public Health Service grants R01-AI070827 and R03-AI085226 (S.L.U.), the American Cancer Society Research Scholar Grant RSG-09-076-01 (S.L.U.), the UIC Center for Clinical and Translational Science NIH grant UL1RR029879, the UIC Council to Support Gastrointestinal and Liver Disease and a grant from the Ministry of Education, Culture, Sports, Science and Technology-Japan, Ministry of Health, Labour and Welfare-Japan (K.C.).
Author information
Authors and Affiliations
Contributions
B.S. made the initial discovery. B.S. and S.L.U. designed the project, analyzed the results and wrote the manuscript. B.S., N.B., D.N.M., S.H., K.A.M. and X.Y. performed experimental work. B.S., S.L.U., M.I. and K.C. designed the hepatic xenorepopulation mouse experiments, and N.H. performed the in vivo studies. W.A.A. was involved in the initial conception of the project and provided valuable expertise.
Corresponding authors
Ethics declarations
Competing interests
B.S. and S.L.U. declare a competing financial interest in the form of a patent application to the US Patent Office entitled "Methods for treatment of hepatitis C" (Appl. No. 61/093,549; filed September 2, 2008 and Appl. No. 61/169,899; filed April 16, 2009), which, in part, is based on and related to the findings described within the manuscript.
Supplementary information
Supplementary Text and Figures
Supplementary Methods and Supplementary Figures 1–13 (PDF 3917 kb)
Rights and permissions
About this article
Cite this article
Sainz, B., Barretto, N., Martin, D. et al. Identification of the Niemann-Pick C1–like 1 cholesterol absorption receptor as a new hepatitis C virus entry factor. Nat Med 18, 281–285 (2012). https://doi.org/10.1038/nm.2581
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2581