Abstract
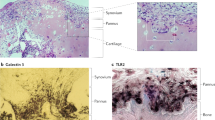
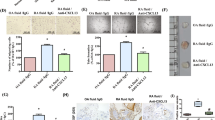
Active rheumatoid arthritis originates from few joints but subsequently affects the majority of joints. Thus far, the pathways of the progression of the disease are largely unknown. As rheumatoid arthritis synovial fibroblasts (RASFs) which can be found in RA synovium are key players in joint destruction and are able to migrate in vitro, we evaluated the potential of RASFs to spread the disease in vivo. To simulate the primary joint of origin, we implanted healthy human cartilage together with RASFs subcutaneously into severe combined immunodeficient (SCID) mice. At the contralateral flank, we implanted healthy cartilage without cells. RASFs showed an active movement to the naive cartilage via the vasculature independent of the site of application of RASFs into the SCID mouse, leading to a marked destruction of the target cartilage. These findings support the hypothesis that the characteristic clinical phenomenon of destructive arthritis spreading between joints is mediated, at least in part, by the transmigration of activated RASFs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Karouzakis, E., Neidhart, M., Gay, R.E. & Gay, S. Molecular and cellular basis of rheumatoid joint destruction. Immunol. Lett. 106, 8–13 (2006).
Müller-Ladner, U., Pap, T., Gay, R.E., Neidhart, M. & Gay, S. Mechanisms of disease: the molecular and cellular basis of joint destruction in rheumatoid arthritis. Nat. Clin. Pract. Rheumatol. 1, 102–110 (2005).
Pap, T., Muller-Ladner, U., Gay, R.E. & Gay, S. Fibroblast biology. Role of synovial fibroblasts in the pathogenesis of rheumatoid arthritis. Arthritis Res. 2, 361–367 (2000).
Gravallese, E.M. Bone destruction in arthritis. Ann. Rheum. Dis. 61 Suppl 2, ii84–ii86 (2002).
Looney, R.J. B cell–targeted therapy for rheumatoid arthritis: an update on the evidence. Drugs 66, 625–639 (2006).
Ma, Y. & Pope, R.M. The role of macrophages in rheumatoid arthritis. Curr. Pharm. Des. 11, 569–580 (2005).
Skapenko, A., Leipe, J., Lipsky, P.E. & Schulze-Koops, H. The role of the T cell in autoimmune inflammation. Arthritis Res. Ther. 7 Suppl 2, S4–S14 (2005).
Yasuda, T. Cartilage destruction by matrix degradation products. Mod. Rheumatol. 16, 197–205 (2006).
Pap, T., Meinecke, I., Muller-Ladner, U. & Gay, S. Are fibroblasts involved in joint destruction? Ann. Rheum. Dis. 64 Suppl 4, iv52–iv54 (2005).
Buckley, C.D. et al. Fibroblasts regulate the switch from acute resolving to chronic persistent inflammation. Trends Immunol. 22, 199–204 (2001).
Huber, L.C. et al. Synovial fibroblasts: key players in rheumatoid arthritis. Rheumatology (Oxford) 45, 669–675 (2006).
Neumann, E. et al. Inhibition of cartilage destruction by double gene transfer of IL-1Ra and IL-10 involves the activin pathway. Gene Ther. 9, 1508–1519 (2002).
Müller-Ladner, U. et al. Synovial fibroblasts of patients with rheumatoid arthritis attach to and invade normal human cartilage when engrafted into SCID mice. Am. J. Pathol. 149, 1607–1615 (1996).
Müller-Ladner, U. et al. Human IL-1Ra gene transfer into human synovial fibroblasts is chondroprotective. J. Immunol. 158, 3492–3498 (1997).
García-Vicuña, R. et al. CC and CXC chemokine receptors mediate migration, proliferation and matrix metalloproteinase production by fibroblast-like synoviocytes from rheumatoid arthritis patients. Arthritis Rheum. 50, 3866–3877 (2004).
Takahara, K. et al. Autocrine/paracrine role of the angiopoietin-1 and -2/Tie2 system in cell proliferation and chemotaxis of cultured fibroblastic synoviocytes in rheumatoid arthritis. Hum. Pathol. 35, 150–158 (2004).
Woods, J.M. et al. A cell-cycle independent role for p21 in regulating synovial fibroblast migration in rheumatoid arthritis. Arthritis Res. Ther. 8, R113 (2006).
van Beurden, H.E., Snoek, P.A., Von den Hoff, J.W., Torensma, R. & Kuijpers-Jagtman, A.M. Fibroblast subpopulations in intra-oral wound healing. Wound Repair Regen. 11, 55–63 (2003).
Clark, R.A.F., An, J.C., Greiling, D., Khan, A. & Schwarzbauer, J.E. Fibroblast migration on fibronectin requires three distinct functional domains. J. Invest. Dermatol. 121, 695–705 (2003).
Blaney Davidson, E.N. et al. Resemblance of osteophytes in experimental osteoarthritis to transforming growth factor β–induced osteophytes. Arthritis Rheum. 56, 4065–4073 (2007).
Zak, J., Schneider, S.W., Eue, I., Ludwig, T. & Oberleithner, H. High-resistance MDCK-C7 monolayers used for measuring invasive potency of tumour cells. Pflugers Arch. 440, 179–183 (2000).
Hutchings, H., Ortega, N. & Plouet, J. Extracellular matrix-bound vascular endothelial growth factor promotes endothelial cell adhesion, migration and survival through integrin ligation. FASEB J. 17, 1520–1522 (2003).
Hubbell, J.A. Matrix-bound growth factors in tissue repair. Swiss Med. Wkly. 137 Suppl 155, 72S–76S (2007).
Hall, H. & Hubbell, J.A. Matrix-bound siwth Ig-like domain of cell adhesion molecule L1 acts as an angiogenic factor by ligating αvβ3-integrin and activating VEGF-R2. Microvasc. Res. 68, 169–178 (2004).
Cazes, A. et al. Extracellular matrix-bound angiopoietin-like 4 inhibits endothelial cell adhesion, migration and sprouting and alters actin cytoskeleton. Circ. Res. 99, 1207–1215 (2006).
Felix, R. et al. Synthesis of membrane- and matrix-bound colony-stimulating factor-1 by cultured osteoblasts. J. Cell. Physiol. 166, 311–322 (1996).
Nicosia, R.F. & Tuszynski, G.P. Matrix-bound thrombospondin promotes angiogenesis in vitro. J. Cell Biol. 124, 183–193 (1994).
Saint-Geniez, M., Kurihara, T. & D'Amore, P.A. Absence of cell and matrix-bound VEGF isoforms is associated with abnormal lens development. Invest. Ophthalmol. Vis. Sci. 50, 311–321 (2009).
el-Gabalawy, H. et al. Synovial distribution of α d/CD18, a novel leukointegrin. Comparison with other integrins and their ligands. Arthritis Rheum. 39, 1913–1921 (1996).
Morales-Ducret, J. et al. α4/β1 integrin (VLA-4) ligands in arthritis. Vascular cell adhesion molecule-1 expression in synovium and on fibroblast-like synoviocytes. J. Immunol. 149, 1424–1431 (1992).
Müller-Ladner, U. et al. Alternatively spliced CS-1 fibronectin isoform and its receptor VLA-4 in rheumatoid arthritis synovium. J. Rheumatol. 24, 1873–1880 (1997).
Schedel, J. et al. Differential adherence of osteoarthritis and rheumatoid arthritis synovial fibroblasts to cartilage and bone matrix proteins and its implication for osteoarthritis pathogenesis. Scand. J. Immunol. 60, 514–523 (2004).
Giancotti, F.G. & Ruoslahti, E. Integrin signaling. Science 285, 1028–1032 (1999).
Owsianik, W.D. et al. Radiological articular involvement in the dominant hand in rheumatoid arthritis. Ann. Rheum. Dis. 39, 508–10 (1980).
Ishikawa, H., Ohno, O. & Hirohata, K. An electron microscopic study of the synovial-bone junction in rheumatoid arthritis. Rheumatol. Int. 4, 1–8 (1984).
Bromley, M., Bertfield, H., Evanson, J.M. & Woolley, D.E. Bidirectional erosion of cartilage in the rheumatoid knee joint. Ann. Rheum. Dis. 44, 676–681 (1985).
Bresnihan, B. Pathogenesis of joint damage in rheumatoid arthritis. J. Rheumatol. 26, 717–719 (1999).
Arnett, F.C. et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 31, 315–324 (1988).
Müller-Ladner, U. et al. Gene transfer of cytokine inhibitors into human synovial fibroblasts in the SCID mouse model. Arthritis Rheum. 42, 490–497 (1999).
Neumann, E. et al. Identification of differentially expressed genes in rheumatoid arthritis by a combination of complementary DNA array and RNA arbitrarily primed-polymerase chain reaction. Arthritis Rheum. 46, 52–63 (2002).
Lechman, E.R. et al. Direct adenoviral gene transfer of viral IL-10 to rabbit knees with experimental arthritis ameliorates disease in both injected and contralateral control knees. J. Immunol. 163, 2202–2208 (1999).
Judex, M. et al. 'Inverse wrap'—an improved implantation technique for virus-transduced synovial fibroblasts in the SCID-mouse model for RA. Mod. Rheumatol. 11, 145–150 (2001).
Clements, K.M., Bee, Z.C., Crossingham, G.V., Adams, M.A. & Sharif, M. How severe must repetitive loading be to kill chondrocytes in articular cartilage? Osteoarthritis Cartilage 9, 499–507 (2001).
Knedla, A. et al. The therapeutic use of osmotic minipumps in the SCID mouse model for rheumatoid arthritis. Ann. Rheum. Dis. 68, 124–129 (2009).
Hashimoto, A. et al. Laser-mediated microdissection for analysis of gene expression in synovial tissue. Mod. Rheumatol. 17, 185–190 (2007).
Judex, M., Neumann, E., Gay, S. & Muller-Ladner, U. Laser-mediated microdissection as a tool for molecular analysis in arthritis. Methods Mol. Med. 101, 93–105 (2004).
Lechner, S. et al. Gene expression pattern of laser microdissected colonic crypts of adenomas with low grade dysplasia. Gut 52, 1148–1153 (2003).
Little, C.B. et al. ADAMTS-1–knockout mice do not exhibit abnormalities in aggrecan turnover in vitro or in vivo. Arthritis Rheum. 52, 1461–1472 (2005).
van der Laan, W.H. et al. Cartilage degradation and invasion by rheumatoid synovial fibroblasts is inhibited by gene transfer of a cell surface-targeted plasmin inhibitor. Arthritis Rheum. 43, 1710–1718 (2000).
Hajiioannou, J.K. et al. In vitro enzymatic treatment and carbon dioxide laser beam irradiation of morphologic cartilage specimens. Arch. Otolaryngol. Head Neck Surg. 132, 1363–1370 (2006).
Acknowledgements
This study was funded by a start-up grant of the German Society of Rheumatology, by research grants of the German Research Foundation (Deutsche Forschungsgemeinschaft: NE1174/3-1, MU1383/14-1, FOR 696), by the Swiss National Fond 32000-116842 and by the Sixth Framework Programme Autocure and Seventh Framework Programme Masterswitch of the EU initiatives. We wish to thank S. Benninghoff, B. Riepl, S. Brückmann and C. Schreiyäck for technical assistance.
Author information
Authors and Affiliations
Contributions
S.L., experiment selection, design and performance, manuscript preparation; A. Knedla, SCID mouse surgery and evaluation; C.T., detection and evaluation of RASFs in mice; A. Kampmann, LMM and evaluation of integrins; C.W., TEER assay and evaluation; R.D., collagenase injection and evaluation; A. Korb, TEER adhesion assay and evaluation; E.-M.S., TEER assay and evaluation; I.H.T., SCID mouse surgery; P.D.R. and C.H.E., preparation of adenoviral vectors; H.S., orthopedic surgery and collection for research; J. Steinmeyer, tissue preparation for experiments; S.G., project design and experimental design; J. Schölmerich, project and experimental design; T.P., project and experimental design, TEER and adhesion assay; U.M.-L., project development and design, experimental design and manuscript preparation; E.N., project development and coordination, study and experimental design and performance and manuscript preparation,
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 (PDF 394 kb)
Rights and permissions
About this article
Cite this article
Lefèvre, S., Knedla, A., Tennie, C. et al. Synovial fibroblasts spread rheumatoid arthritis to unaffected joints. Nat Med 15, 1414–1420 (2009). https://doi.org/10.1038/nm.2050
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2050