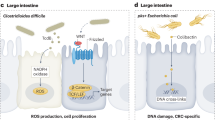
Abstract
The intestinal flora may promote colon tumor formation. Here we explore immunologic mechanisms of colonic carcinogenesis by a human colonic bacterium, enterotoxigenic Bacteroides fragilis (ETBF). ETBF that secretes B. fragilis toxin (BFT) causes human inflammatory diarrhea but also asymptomatically colonizes a proportion of the human population. Our results indicate that whereas both ETBF and nontoxigenic B. fragilis (NTBF) chronically colonize mice, only ETBF triggers colitis and strongly induces colonic tumors in multiple intestinal neoplasia (Min) mice. ETBF induces robust, selective colonic signal transducer and activator of transcription-3 (Stat3) activation with colitis characterized by a selective T helper type 17 (TH17) response distributed between CD4+ T cell receptor-αβ (TCRαβ)+ and CD4–8–TCRγδ+ T cells. Antibody-mediated blockade of interleukin-17 (IL-17) as well as the receptor for IL-23, a key cytokine amplifying TH17 responses, inhibits ETBF-induced colitis, colonic hyperplasia and tumor formation. These results show a Stat3- and TH17-dependent pathway for inflammation-induced cancer by a common human commensal bacterium, providing new mechanistic insight into human colon carcinogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Suerbaum, S. & Michetti, P. Helicobacter pylori infection. N. Engl. J. Med. 347, 1175–1186 (2002).
El Serag, H.B. & Rudolph, K.L. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 132, 2557–2576 (2007).
Greten, F.R. et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118, 285–296 (2004).
Karin, M. & Greten, F.R. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 5, 749–759 (2005).
Naugler, W.E. et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 317, 121–124 (2007).
Naugler, W.E. & Karin, M. The wolf in sheep's clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol. Med. 14, 109–119 (2008).
Yu, H. & Jove, R. The STATs of cancer—new molecular targets come of age. Nat. Rev. Cancer 4, 97–105 (2004).
Yu, H., Kortylewski, M. & Pardoll, D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat. Rev. Immunol. 7, 41–51 (2007).
Erdman, S.E. et al. CD4+ CD25+ regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice. Am. J. Pathol. 162, 691–702 (2003).
Dunn, G.P., Koebel, C.M. & Schreiber, R.D. Interferons, immunity and cancer immunoediting. Nat. Rev. Immunol. 6, 836–848 (2006).
Weaver, C.T., Hatton, R.D., Mangan, P.R. & Harrington, L.E. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 25, 821–852 (2007).
Laurence, A. & O'Shea, J.J. TH-17 differentiation: of mice and men. Nat. Immunol. 8, 903–905 (2007).
Cho, J.H. The genetics and immunopathogenesis of inflammatory bowel disease. Nat. Rev. Immunol. 8, 458–466 (2008).
Rakoff-Nahoum, S. & Medzhitov, R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science 317, 124–127 (2007).
Su, L.K. et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science 256, 668–670 (1992).
Hope, M.E., Hold, G.L., Kain, R. & El Omar, E.M. Sporadic colorectal cancer–role of the commensal microbiota. FEMS Microbiol. Lett. 244, 1–7 (2005).
Sears, C.L. et al. Enterotoxigenic Bacteroides fragilis infection is associated with inflammatory diarrhea. Clin. Infect. Dis. 47, 797–803 (2008).
Toprak, N.U. et al. A possible role of Bacteroides fragilis enterotoxin in the aetiology of colorectal cancer. Clin. Microbiol. Infect. 12, 782–786 (2006).
Levy, D.E. & Darnell, J.E., Jr. Stats: transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 3, 651–662 (2002).
Chen, Z. et al. Selective regulatory function of Socs3 in the formation of IL-17–secreting T cells. Proc. Natl. Acad. Sci. USA 103, 8137–8142 (2006).
Laurence, A. et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity 26, 371–381 (2007).
Harris, T.J. et al. Cutting edge: An in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J. Immunol. 179, 4313–4317 (2007).
Ivanov, I.I. et al. The orphan nuclear receptor RORγat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006).
Kortylewski, M. et al. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell 15, 114–123 (2009).
Langowski, J.L. et al. IL-23 promotes tumour incidence and growth. Nature 442, 461–465 (2006).
Bollrath, J. et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell 15, 91–102 (2009).
Grivennikov, S. et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 15, 103–113 (2009).
de Visser, K.E., Korets, L.V. & Coussens, L.M. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell 7, 411–423 (2005).
Poutahidis, T. et al. Rapid reversal of interleukin-6–dependent epithelial invasion in a mouse model of microbially induced colon carcinoma. Carcinogenesis 28, 2614–2623 (2007).
Rao, V.P. et al. Proinflammatory CD4+ CD45RBhi lymphocytes promote mammary and intestinal carcinogenesis in ApcMin/+ mice. Cancer Res. 66, 57–61 (2006).
Müller-Hermelink, N. et al. TNFR1 signaling and IFN-γ signaling determine whether T cells induce tumor dormancy or promote multistage carcinogenesis. Cancer Cell 13, 507–518 (2008).
Koebel, C.M. et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature 450, 903–907 (2007).
Mangan, P.R. et al. Transforming growth factor-β induces development of the TH17 lineage. Nature 441, 231–234 (2006).
Kullberg, M.C. et al. IL-23 plays a key role in Helicobacter hepaticus–induced T cell–dependent colitis. J. Exp. Med. 203, 2485–2494 (2006).
Hue, S. et al. Interleukin-23 drives innate and T cell–mediated intestinal inflammation. J. Exp. Med. 203, 2473–2483 (2006).
Newman, J.V., Kosaka, T., Sheppard, B.J., Fox, J.G. & Schauer, D.B. Bacterial infection promotes colon tumorigenesis in ApcMin/+ mice. J. Infect. Dis. 184, 227–230 (2001).
Nagamine, C.M. et al. Helicobacter hepaticus infection promotes colon tumorigenesis in the BALB/c-Rag2−/−ApcMin/+ mouse. Infect. Immun. 76, 2758–2766 (2008).
Maggio-Price, L. et al. Helicobacter infection is required for inflammation and colon cancer in SMAD3-deficient mice. Cancer Res. 66, 828–838 (2006).
Raffatellu, M. et al. Simian immunodeficiency virus–induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat. Med. 14, 421–428 (2008).
Niess, J.H., Leithauser, F., Adler, G. & Reimann, J. Commensal gut flora drives the expansion of proinflammatory CD4 T cells in the colonic lamina propria under normal and inflammatory conditions. J. Immunol. 180, 559–568 (2008).
Wu, S., Lim, K.-C., Huang, J., Saidi, R.F. & Sears, C.L. Bacteroides fragilis enterotoxin cleaves the zonula adherens protein, E-cadherin. Proc. Natl. Acad. Sci. USA 95, 14979–14984 (1998).
Wu, S., Morin, P.J., Maouyo, D. & Sears, C.L. Bacteroides fragilis enterotoxin induces c-Myc expression and cellular proliferation. Gastroenterology 124, 392–400 (2003).
Sears, C.L. Enterotoxigenic Bacteroides fragilis: a rogue among symbiotes. Clin. Microbiol. Rev. 22, 349–369 (2009).
Rhee, K.J. et al. Induction of persistent colitis by a human commensal, enterotoxigenic Bacteroides fragilis, in wild-type C57BL/6 mice. Infect. Immun. 77, 1708–1718 (2009).
Caruso, R. et al. IL-23–mediated regulation of IL-17 production in Helicobacter pylori–infected gastric mucosa. Eur. J. Immunol. 38, 470–478 (2008).
Boivin, G.P. et al. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology 124, 762–777 (2003).
Acknowledgements
This work was supported by the Crohn's and Colitis Foundation through a Senior Investigator Award (to C.L.S.) and a Research Fellowship Award (to K.-J.R.), RO1 DK45496 (to C.L.S.), RO1 DK080817 (to C.L.S.), US National Institutes of Health grants (to D.M.P.), Special Projects of Research Excellence grant CA62924, R24 DK64388 (to M. Donowitz, the principal investigator of this grant that provided resource support to this project), RR00171 grant (to D.L.H.), Institutional Training for Pediatricians 5 T32 HD44355 (to G. Dover, the principal investigator of this grant that provided partial salary support to S.R.), Clinical Pharmacology Training Program 2 T32GM066691 (to T. Shapiro, the principal investigator of this grant that provided salary support to F.M.) and F32 DK079509 (to S.R.). This work was also supported by gifts from B. Schwartz, W. and B. Topercer, D. Needle, B. Swartz and the Commonwealth Foundation. D.M.P. is a Januey scholar and holds the Abeloff Chair in Oncology at Johns Hopkins University. We thank J. Wolfe for her assistance with some experiments; L. Myers (formerly Montana State University) for ETBF strain 86-5443-2-2; B. Vogelstein and K. Kinzler (Johns Hopkins University School of Medicine) for Min mice and E. Jaffee (Johns Hopkins University School of Medicine) for GK1.5 antibody.
Author information
Authors and Affiliations
Contributions
S.W. and K.-J.R. performed the majority of tumorigenesis experiments. E.A., S.R. and E.W. performed Stat experiments. X.W. did most of the mouse breeding and assisted with experiments. H.-R.Y. assisted with conditional CD4 Stat3-KO mouse experiments. D.L.H. evaluated and interpreted the histopathology. F.L.B. contributed the statistical analyses. F.M. performed qRT-PCR experiments. F.H. provided oversight and strategic planning for colonic immunology analyses. D.M.P. and C.L.S. designed the study, reviewed and discussed experiments and wrote the manuscript with input from co-authors.
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figs.1–6 and Supplementary Tables 1 and 2 (PDF 3375 kb)
Rights and permissions
About this article
Cite this article
Wu, S., Rhee, KJ., Albesiano, E. et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med 15, 1016–1022 (2009). https://doi.org/10.1038/nm.2015
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2015
This article is cited by
-
Bacteroides fragilis toxin expression enables lamina propria niche acquisition in the developing mouse gut
Nature Microbiology (2024)
-
The microbiota and renal cell carcinoma
Cellular Oncology (2024)
-
Role of Gut Microbiota in Predisposition to Colon Cancer: A Narrative Review
Indian Journal of Microbiology (2024)
-
Intratumoral presence of the genotoxic gut bacteria pks+ E. coli, Enterotoxigenic Bacteroides fragilis, and Fusobacterium nucleatum and their association with clinicopathological and molecular features of colorectal cancer
British Journal of Cancer (2024)
-
Identification of FtfL as a novel target of berberine in intestinal bacteria
BMC Biology (2023)