Abstract
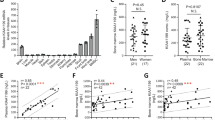
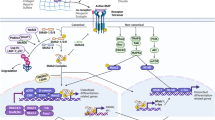
Bone remodeling depends on the precise coordination of bone resorption and subsequent bone formation. Disturbances of this process are associated with skeletal diseases, such as Camurati-Engelmann disease (CED). We show using in vitro and in vivo models that active TGF-β1 released during bone resorption coordinates bone formation by inducing migration of bone marrow stromal cells, also known as bone mesenchymal stem cells, to the bone resorptive sites and that this process is mediated through a SMAD signaling pathway. Analyzing mice carrying a CED-derived mutant TGFB1 (encoding TGF-β1), which show the typical progressive diaphyseal dysplasia seen in the human disease, we found high levels of active TGF-β1 in the bone marrow. Treatment with a TGF-β type I receptor inhibitor partially rescued the uncoupled bone remodeling and prevented the fractures. Thus, as TGF-β1 functions to couple bone resorption and formation, modulation of TGF-β1 activity could be an effective treatment for bone remodeling diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Zaidi, M. Skeletal remodeling in health and disease. Nat. Med. 13, 791–801 (2007).
Hill, P.A. Bone remodelling. Br. J. Orthod. 25, 101–107 (1998).
Janssens, K. et al. Mutations in the gene encoding the latency-associated peptide of TGF-beta 1 cause Camurati-Engelmann disease. Nat. Genet. 26, 273–275 (2000).
Kinoshita, A. et al. Domain-specific mutations in TGFB1 result in Camurati-Engelmann disease. Nat. Genet. 26, 19–20 (2000).
Hecht, J.T. et al. Evidence for locus heterogeneity in the Camurati-Engelmann (DPD1) syndrome. Clin. Genet. 59, 198–200 (2001).
Oreffo, R.O., Mundy, G.R., Seyedin, S.M. & Bonewald, L.F. Activation of the bone-derived latent TGF beta complex by isolated osteoclasts. Biochem. Biophys. Res. Commun. 158, 817–823 (1989).
Mundy, G.R. Peptides and growth regulatory factors in bone. Rheum. Dis. Clin. North Am. 20, 577–588 (1994).
Martin, T.J., Allan, E.H. & Fukumoto, S. The plasminogen-activator and inhibitor system in bone remodeling. Growth Regul. 3, 209–214 (1993).
Hill, P.A., Tumber, A. & Meikle, M.C. Multiple extracellular signals promote osteoblast survival and apoptosis. Endocrinology 138, 3849–3858 (1997).
Pfeilschifter, J. et al. Chemotactic response of osteoblast-like cells to transforming growth-factor-beta. J. Bone Miner. Res. 5, 825–830 (1990).
Linkhart, T.A., Mohan, S. & Baylink, D.J. Growth factors for bone growth and repair: IGF, TGF beta and BMP. Bone 19, 1S–12S (1996).
Hughes, F.J., Aubin, J.E. & Heersche, J.N.M. Differential chemotactic responses of different populations of fetal-rat calvaria cells to platelet-derived growth-factor and transforming growth-factor-beta. Bone Miner. 19, 63–74 (1992).
Bismar, H. et al. Transforming growth factor beta (TGF-beta) levels in the conditioned media of human bone cells: relationship to donor age, bone volume, and concentration of TGF-beta in human bone matrix in vivo. Bone 24, 565–569 (1999).
Roberts, A.B., Frolik, C.A., Anzano, M.A. & Sporn, M.B. Transforming growth factors from neoplastic and nonneoplastic tissues. Fed. Proc. 42, 2621–2626 (1983).
Seyedin, S.M., Thomas, T.C., Thompson, A.Y., Rosen, D.M. & Piez, K.A. Purification and characterization of two cartilage-inducing factors from bovine demineralized bone. Proc. Natl. Acad. Sci. USA 82, 2267–2271 (1985).
Dallas, S.L. et al. Characterization and autoregulation of latent transforming growth-factor-beta (TGF-beta) complexes in osteoblast-like cell-lines - production of a latent complex lacking the latent TGF-beta-binding protein. J. Biol. Chem. 269, 6815–6821 (1994).
Gentry, L.E., Lioubin, M.N., Purchio, A.F. & Marquardt, H. Molecular events in the processing of recombinant type 1 pre-pro-transforming growth factor beta to the mature polypeptide. Mol. Cell. Biol. 8, 4162–4168 (1988).
Pfeilschifter, J., Bonewald, L. & Mundy, G.R. Characterization of the latent transforming growth factor beta complex in bone. J. Bone Miner. Res. 5, 49–58 (1990).
Pedrozo, H.A. et al. Potential mechanisms for the plasmin-mediated release and activation of latent transforming growth factor-beta 1 from the extracellular matrix of growth plate chondrocytes. Endocrinology 140, 5806–5816 (1999).
Janssens, K., ten Dijke, P., Janssens, S. & Van Hul, W. Transforming growth factor-beta 1 to the bone. Endocr. Rev. 26, 743–774 (2005).
Jian, H. et al. Smad3-dependent nuclear translocation of beta-catenin is required for TGF-beta 1-induced proliferation of bone marrow-derived adult human mesenchymal stem cells. Genes Dev. 20, 666–674 (2006).
Erlebacher, A., Filvaroff, E.H., Ye, J.Q. & Derynck, R. Osteoblastic responses to TGF-beta during bone remodeling. Mol. Biol. Cell 9, 1903–1918 (1998).
Filvaroff, E. et al. Inhibition of TGF-beta receptor signaling in osteoblasts leads to decreased bone remodeling and increased trabecular bone mass. Development 126, 4267–4279 (1999).
Janssens, K. et al. Camurati-Engelmann disease: review of the clinical, radiological, and molecular data of 24 families and implications for diagnosis and treatment. J. Med. Genet. 43, 1–11 (2006).
Janssens, K., ten Dijke, P., Ralston, S.H., Bergmann, C. & Van Hul, W. Transforming growth factor-beta 1 mutations in Camurati-Engelmann disease lead to increased signaling by altering either activation or secretion of the mutant protein. J. Biol. Chem. 278, 7718–7724 (2003).
Saito, T. et al. Domain-specific mutations of a transforming growth factor (TGF)-beta 1 latency-associated peptide cause Camurati-Engelmann disease because of the formation of a constitutively active form of TGF-beta 1. J. Biol. Chem. 276, 11469–11472 (2001).
Prockop, D.J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276, 71–74 (1997).
Caplan, A.I. Mesenchymal stem-cells. J. Orthop. Res. 9, 641–650 (1991).
Dennis, J.E., Carbillet, J.P., Caplan, A.I. & Charbord, P. The STRO-1+ marrow cell population is multipotential. Cells Tissues Organs 170, 73–82 (2002).
Gronthos, S., Graves, S.E., Ohta, S. & Simmons, P.J. The Stro-1+ fraction of adult human bone-marrow contains the osteogenic precursors. Blood 84, 4164–4173 (1994).
Sacchetti, B. et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131, 324–336 (2007).
Meirelles, L.S. & Nardi, N.B. Murine marrow-derived mesenchymal stem cell: isolation, in vitro expansion, and characterization. Br. J. Haematol. 123, 702–711 (2003).
Leucht, P. et al. Effect of mechanical stimuli on skeletal regeneration around implants. Bone 40, 919–930 (2007).
Caplan, A.I. Mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 11, 1198–1211 (2005).
Horwitz, E.M. et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat. Med. 5, 309–313 (1999).
Horwitz, E.M. et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc. Natl. Acad. Sci. USA 99, 8932–8937 (2002).
Mirmalek-Sani, S.H. et al. Characterization and multipotentiality of human fetal femur-derived cells: implications for skeletal tissue regeneration. Stem Cells 24, 1042–1053 (2006).
Canalis, E., Economides, A.N. & Gazzerro, E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr. Rev. 24, 218–235 (2003).
Ozaki, Y. et al. Comprehensive analysis of chemotactic factors for bone marrow mesenchymal stem cells. Stem Cells Dev. 16, 119–130 (2007).
Maeda, S., Hayashi, M., Komiya, S., Imamura, T. & Miyazono, K. Endogenous TGF-beta signaling suppresses maturation of osteoblastic mesenchymal cells. EMBO J. 23, 552–563 (2004).
Kulkarni, A.B. et al. Transforming growth factor-beta-1 null mutation in mice causes excessive inflammatory response and early death. Proc. Natl. Acad. Sci. USA 90, 770–774 (1993).
Geiser, A.G. et al. Decreased bone mass and bone elasticity in mice lacking the transforming growth factor-beta 1 gene. Bone 23, 87–93 (1998).
Atti, E. et al. Effects of transforming growth factor-beta deficiency on bone development: a Fourier transform-infrared imaging analysis. Bone 31, 675–684 (2002).
Shinkai, Y. et al. Rag-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68, 855–867 (1992).
Engle, S.J. et al. Elimination of colon cancer in germ-free transforming growth factor beta 1-deficient mice. Cancer Res. 62, 6362–6366 (2002).
Massagué, J. TGF-beta signal transduction. Annu. Rev. Biochem. 67, 753–791 (1998).
Attisano, L. & Wrana, J.L. Signal transduction by the TGF-beta superfamily. Science 296, 1646–1647 (2002).
DaCosta Byfield, S., Major, C., Laping, N.J. & Roberts, A.B. SB-505124 is a selective inhibitor of transforming growth factor-beta type I receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 65, 744–752 (2004).
Hayashi, H. et al. The MAD-related protein Smad7 associates with the TGF beta receptor and functions as an antagonist of TGF beta signaling. Cell 89, 1165–1173 (1997).
Yang, X., Li, C.L., Herrera, P.L. & Deng, C.X. Generation of Smad4/Dpc4 conditional knockout mice. Genesis 32, 80–81 (2002).
Engler, A.J., Sen, S., Sweeney, H.L. & Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006).
Zink, A.R. et al. Evidence for a 7000-year-old case of primary hyperparathyroidism. JAMA 293, 40–42 (2005).
Roodman, G.D. & Windle, J.J. Paget disease of bone. J. Clin. Invest. 115, 200–208 (2005).
Surks, M.I. et al. Subclinical thyroid disease - Scientific review and guidelines for diagnosis and management. JAMA 291, 228–238 (2004).
Friedenstein, A.J., Chailakhyan, R.K. & Gerasimov, U.V. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 20, 263–272 (1987).
Owen, M. & Friedenstein, A.J. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found. Symp. 136, 42–60 (1988).
Friedenstein, A.J. et al. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp. Hematol. 2, 83–92 (1974).
Acknowledgements
The authors thank F. Hunter and T. Clemens for critical review and discussion of the manuscript and H.L. Moses (Vanderbilt University), C. Deng (US National Institutes of Health), J. Murphy-Ullrich (University of Alabama at Birmingham) and W. Xiong (Medical College of Georgia) for reagents. We thank the UAB Center for Metabolic Bone Disease Cores for mouse phenotyping, histomorphometry and molecular analyses. This research was supported by US National Institutes of Health grants AR 053973 and DK057501.
Author information
Authors and Affiliations
Contributions
Y.T. and X.W. performed the majority of the experiments, analyzed data and prepared the manuscript. W.L. maintained mice and collected tissue samples. L.P. helped with the in vitro Transwell migration assay. C.W. helped with μCT analyses. Z.S. helped with the in vitro bone resorption assay. L.Z. assisted with in vivo experiments. T.R.N. helped with X-ray analyses. X.P., J.H., X.F. and W.V.H. provided suggestions for the project. M.W. prepared the manuscript. X.C. supervised the project and wrote most of the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6, Supplementary Table 1 and Supplementary Methods (PDF 990 kb)
Rights and permissions
About this article
Cite this article
Tang, Y., Wu, X., Lei, W. et al. TGF-β1–induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med 15, 757–765 (2009). https://doi.org/10.1038/nm.1979
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.1979
This article is cited by
-
The roles and regulatory mechanisms of TGF-β and BMP signaling in bone and cartilage development, homeostasis and disease
Cell Research (2024)
-
The emerging studies on mesenchymal progenitors in the long bone
Cell & Bioscience (2023)
-
Re-thinking osteoarthritis pathogenesis: what can we learn (and what do we need to unlearn) from mouse models about the mechanisms involved in disease development
Arthritis Research & Therapy (2023)
-
Targeting strategies for bone diseases: signaling pathways and clinical studies
Signal Transduction and Targeted Therapy (2023)
-
Atp6i deficient mouse model uncovers transforming growth factor-β1 /Smad2/3 as a key signaling pathway regulating odontoblast differentiation and tooth root formation
International Journal of Oral Science (2023)