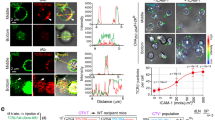
Abstract
Activated T helper cells produce many cytokines, some of which are secreted through the immunological synapse toward the antigen-presenting cell. Here we have used immunocytochemistry, live-cell imaging and a surface-mediated secretion assay to show that there are two cytokine export pathways in T helper cells. Some cytokines, including interleukin 2 and interferon-γ, were secreted into the synapse, whereas others, including tumor necrosis factor and the chemokine CCL3 (MIP-1α), were released multidirectionally. Each secretion pathway was associated with different trafficking proteins, indicating that they are molecularly distinct processes. These data suggest that T helper cells release some cytokines into the immunological synapse to impart specific communication and others multidirectionally to promote inflammation and to establish chemokine gradients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Bromley, S.K. et al. The immunological synapse. Annu. Rev. Immunol. 19, 375–396 (2001).
Monks, C.R., Freiberg, B.A., Kupfer, H., Sciaky, N. & Kupfer, A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 395, 82–86 (1998).
Grakoui, A. et al. The immunological synapse: a molecular machine controlling T cell activation. Science 285, 221–227 (1999).
Sancho, D. et al. Regulation of microtubule-organizing center orientation and actomyosin cytoskeleton rearrangement during immune interactions. Immunol. Rev. 189, 84–97 (2002).
Kupfer, A. & Singer, S.J. Cell biology of cytotoxic and helper T cell functions: immunofluorescence microscopic studies of single cells and cell couples. Annu. Rev. Immunol. 7, 309–337 (1989).
Davis, S.J. & van der Merwe, P.A. The immunological synapse: required for T cell receptor signalling or directing T cell effector function? Curr. Biol. 11, R289–R291 (2001).
Stinchcombe, J.C., Bossi, G., Booth, S. & Griffiths, G.M. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity 15, 751–761 (2001).
Kupfer, A., Mosmann, T.R. & Kupfer, H. Polarized expression of cytokines in cell conjugates of helper T cells and splenic B cells. Proc. Natl. Acad. Sci. USA 88, 775–779 (1991).
Kupfer, H., Monks, C.R. & Kupfer, A. Small splenic B cells that bind to antigen-specific T helper (Th) cells and face the site of cytokine production in the Th cells selectively proliferate: immunofluorescence microscopic studies of Th-B antigen-presenting cell interactions. J. Exp. Med. 179, 1507–1515 (1994).
Reichert, P., Reinhardt, R.L., Ingulli, E. & Jenkins, M.K. Cutting edge: in vivo identification of TCR redistribution and polarized IL-2 production by naive CD4 T cells. J. Immunol. 166, 4278–4281 (2001).
Poo, W.J., Conrad, L. & Janeway, C.A., Jr. Receptor-directed focusing of lymphokine release by helper T cells. Nature 332, 378–380 (1988).
Pagan, J.K. et al. The t-SNARE syntaxin 4 is regulated during macrophage activation to function in membrane traffic and cytokine secretion. Curr. Biol. 13, 156–160 (2003).
Bock, J.B., Matern, H.T., Peden, A.A. & Scheller, R.H. A genomic perspective on membrane compartment organization. Nature 409, 839–841 (2001).
Segev, N. Ypt and Rab GTPases: insight into functions through novel interactions. Curr. Opin. Cell Biol. 13, 500–511 (2001).
Zerial, M. & McBride, H. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2, 107–117 (2001).
Jahn, R., Lang, T. & Sudhof, T.C. Membrane fusion. Cell 112, 519–533 (2003).
Parlati, F. et al. Distinct SNARE complexes mediating membrane fusion in Golgi transport based on combinatorial specificity. Proc. Natl. Acad. Sci. USA 99, 5424–5429 (2002).
Allenspach, E.J. et al. ERM-dependent movement of CD43 defines a novel protein complex distal to the immunological synapse. Immunity 15, 739–750 (2001).
Morales-Tirado, V. et al. Cutting edge: selective requirement for the Wiskott-Aldrich syndrome protein in cytokine, but not chemokine, secretion by CD4+ T cells. J. Immunol. 173, 726–730 (2004).
Chen, D.S. et al. Marked differences in human melanoma antigen-specific T cell responsiveness following peptide vaccination using a functional microarray. PloS Med. 2, e265 (2005) published online 20 September 2005 (10.1371/journal.pmed.0020265).
Catalfamo, M. et al. Human CD8+ T cells store RANTES in a unique secretory compartment and release it rapidly after TcR stimulation. Immunity 20, 219–230 (2004).
Darchen, F. & Goud, B. Multiple aspects of Rab protein action in the secretory pathway: focus on Rab3 and Rab6. Biochimie 82, 375–384 (2000).
Riedel, D. et al. Rab3D is not required for exocrine exocytosis but for maintenance of normally sized secretory granules. Mol. Cell. Biol. 22, 6487–6497 (2002).
Wendler, F. & Tooze, S. Syntaxin 6: the promiscuous behaviour of a SNARE protein. Traffic 2, 606–611 (2001).
Masuda, E.S. et al. Rab37 is a novel mast cell specific GTPase localized to secretory granules. FEBS Lett. 470, 61–64 (2000).
Antonin, W. et al. A SNARE complex mediating fusion of late endosomes defines conserved properties of SNARE structure and function. EMBO J. 19, 6453–6464 (2000).
Hirst, J., Miller, S.E., Taylor, M.J., von Mollard, G.F. & Robinson, M.S. EpsinR is an adaptor for the SNARE protein Vti1b. Mol. Biol. Cell 15, 5593–5602 (2004).
Chidambaram, S., Mullers, N., Wiederhold, K., Haucke, V. & von Mollard, G.F. Specific interaction between SNAREs and epsin N-terminal homology (ENTH) domains of epsin-related proteins in trans-Golgi network to endosome transport. J. Biol. Chem. 279, 4175–4179 (2004).
Murray, R.Z., Wylie, F.G., Khromykh, T., Hume, D.A. & Stow, J.L. Syntaxin 6 and Vti1b form a novel SNARE complex, which is up-regulated in activated macrophages to facilitate exocytosis of tumor necrosis factor-α. J. Biol. Chem. 280, 10478–10483 (2005).
Huber, L.A., Dupree, P. & Dotti, C.G. A deficiency of the small GTPase rab8 inhibits membrane traffic in developing neurons. Mol. Cell. Biol. 15, 918–924 (1995).
Ang, A.L., Folsch, H., Koivisto, U.M., Pypaert, M. & Mellman, I. The Rab8 GTPase selectively regulates AP-1B-dependent basolateral transport in polarized Madin-Darby canine kidney cells. J. Cell Biol. 163, 339–350 (2003).
Stinchcombe, J.C. et al. Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J. Cell Biol. 152, 825–834 (2001).
Butz, E.A. & Bevan, M.J. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity 8, 167–175 (1998).
Murali-Krishna, K. et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8, 177–187 (1998).
Boehm, U., Klamp, T., Groot, M. & Howard, J.C. Cellular responses to interferon-γ. Annu. Rev. Immunol. 15, 749–795 (1997).
Pestka, S. et al. Interleukin-10 and related cytokines and receptors. Annu. Rev. Immunol. 22, 929–979 (2004).
Grouard, G. et al. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J. Exp. Med. 185, 1101–1111 (1997).
Locksley, R.M., Killeen, N. & Lenardo, M.J. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104, 487–501 (2001).
Sallusto, F., Mackay, C.R. & Lanzavecchia, A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu. Rev. Immunol. 18, 593–620 (2000).
Schmitz, J. et al. Induction of interleukin 4 (IL-4) expression in T helper (Th) cells is not dependent on IL-4 from non-Th cells. J. Exp. Med. 179, 1349–1353 (1994).
Glimcher, L.H. & Murphy, K.M. Lineage commitment in the immune system: the T helper lymphocyte grows up. Genes Dev. 14, 1693–1711 (2000).
Okada, T. et al. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Bio 3, e150 (2005) published online 3 May 2005 (10.1371/journal.pbio.0030150).
Croft, M. & Swain, S.L. B cell response to fresh and effector T helper cells. Role of cognate T-B interaction and the cytokines IL-2, IL-4, and IL-6. J. Immunol. 146, 4055–4064 (1991).
Calderhead, D.M., Kosaka, Y., Manning, E.M. & Noelle, R.J. CD40–CD154 interactions in B-cell signaling. Curr. Top. Microbiol. Immunol. 245, 73–99 (2000).
Boisvert, J., Edmondson, S. & Krummel, M.F. Immunological synapse formation licenses CD40–CD40L accumulations at T-APC contact sites. J. Immunol. 173, 3647–3652 (2004).
Maldonado, R.A., Irvine, D.J., Schreiber, R. & Glimcher, L.H. A role for the immunological synapse in lineage commitment of CD4 lymphocytes. Nature 431, 527–532 (2004).
Taylor, P.C., Williams, R.O. & Feldmann, M. Tumour necrosis factor α as a therapeutic target for immune-mediated inflammatory diseases. Curr. Opin. Biotechnol. 15, 557–563 (2004).
Kontoyiannis, D., Pasparakis, M., Pizarro, T.T., Cominelli, F. & Kollias, G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity 10, 387–398 (1999).
Grivennikov, S.I. et al. Distinct and nonredundant in vivo functions of TNF produced by T cells and macrophages/neutrophils: protective and deleterious effects. Immunity 22, 93–104 (2005).
Steinman, L. Immune therapy for autoimmune diseases. Science 305, 212–216 (2004).
Spiliotis, E.T. & Nelson, W.J. Spatial control of exocytosis. Curr. Opin. Cell Biol. 15, 430–437 (2003).
Boniface, J.J., Reich, Z., Lyons, D.S. & Davis, M.M. Thermodynamics of T cell receptor binding to peptide-MHC: evidence for a general mechanism of molecular scanning. Proc. Natl. Acad. Sci. USA 96, 11446–11451 (1999).
Purbhoo, M.A., Irvine, D.J., Huppa, J.B. & Davis, M.M. T cell killing does not require the formation of a stable mature immunological synapse. Nat. Immunol. 5, 524–530 (2004).
Irvine, D.J., Purbhoo, M.A., Krogsgaard, M. & Davis, M.M. Direct observation of ligand recognition by T cells. Nature 419, 845–849 (2002).
Acknowledgements
We thank L. Steinman, H. McDevitt, W.J. Nelson and K. Pham for discussions and for critical reading of this manuscript; S. Pfeffer for comments; E. Gallo for assistance with the Affymetrix data; L. Klein for advice with colocalization; J. Mulholland and K. Lee for assistance with confocal microscopy; N. Prado and J. Fabian for technical assistance; and other members of the Davis lab for discussions and support. Supported by the Helen Hay Whitney Foundation (M.H.), Giannini Family Foundation (M.H.), Human Frontiers Science Program (B.F.L.), Cancer Research Institute (M.S.K.), National Institutes of Health (M.M.D.) and Howard Hughes Medical Institute (M.M.D.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Gradual appearance of non-synapse associated TNF compartments. (PDF 43 kb)
Supplementary Fig. 2
TAPI-0-mediated blockade of TNF release. (PDF 72 kb)
Supplementary Fig. 3
Stability of TNF puncta over time. (PDF 282 kb)
Supplementary Fig. 4
Intracellular IL-4 has Golgi-associated and Golgi-independent pools. (PDF 109 kb)
Rights and permissions
About this article
Cite this article
Huse, M., Lillemeier, B., Kuhns, M. et al. T cells use two directionally distinct pathways for cytokine secretion. Nat Immunol 7, 247–255 (2006). https://doi.org/10.1038/ni1304
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1304