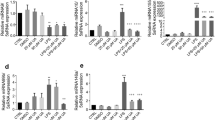
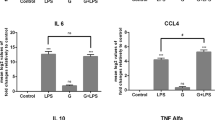
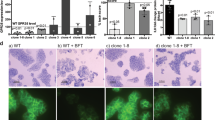
Abstract
The human gut microflora is important in regulating host inflammatory responses and in maintaining immune homeostasis. The cellular and molecular bases of these actions are unknown. Here we describe a unique anti-inflammatory mechanism, activated by nonpathogenic bacteria, that selectively antagonizes transcription factor NF-κB. Bacteroides thetaiotaomicron targets transcriptionally active NF-κB subunit RelA, enhancing its nuclear export through a mechanism independent of nuclear export receptor Crm-1. Peroxisome proliferator activated receptor-γ (PPAR-γ), in complex with nuclear RelA, also undergoes nucleocytoplasmic redistribution in response to B. thetaiotaomicron. A decrease in PPAR-γ abolishes both the nuclear export of RelA and the anti-inflammatory activity of B. thetaiotaomicron. This PPAR-γ-dependent anti-inflammatory mechanism defines new cellular targets for therapeutic drug design and interventions for the treatment of chronic inflammation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Sartor, R.B. The influence of normal microbial flora on the development of chronic inflammation. Res. Immunol. 148, 567–576 (1997).
Rembacken, B.J. et al. Non-pathogenic E. coli versus mesalazine for the treament of ulcerative colitis: a randomised trial. Lancet 354, 635–639 (1999)
Kuhn, R., Lohler, J., Rennick, D., Rajewsky, K. & Muller, W. Interleukin-10 deficient mice develop chronic enterocolitis. Cell 75, 263–274 (1993).
Powrie, F., Correa-Oliveira, R., Mauze, S. & Coffman, R.L. Regulatory interactions between CD45RBhigh and CD45RBlow CD4+ T cells are important for the balance between protective and pathogenic T-cell immunity. J. Exp. Med. 179, 589–600 (1994).
Campieri, M. & Gionchetti, P. Probiotics in inflammatory bowel disease: new insight to pathogenesis or possible therapeutic alternative. Gastroenterol. 116, 1246–1249 (1999).
Marteau, P.R., de Vrese, M., Cellier, C.J. & Schrezenmeir, J. Protection from gastrointestinal diseases with the use of probiotics. Am. J. Clin. Nutr. 73, 430S–436S (2001).
Cario, E. et al. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J. Immunol. 164, 966–972 (2000).
Cong, Y., Weaver, C.T., Lazenby, A. & Elson, C.O. Bacterial-reactive T regulatory cells inhibit pathogenic immune responses to the enteric flora. J. Immunol. 169, 6112–6119 (2002).
Neish, A.S. et al. Prokaryotic regulation of epithelial responses by inhibition of IκB-α ubiquitination. Science 289, 1560–1563 (2000).
Kelly, D. & Conway, S. Genomics at work: the global gene response to enteric bacteria. Gut 49, 612–613 (2001).
McCormick, B.A., Colgan, S.P., Delp-Archer, C., Miller, S.I. & Madara, J.L. Salmonella typhimurium attachment to human intestinal epithelial monolayers: transcellular signalling to subepithelial neutrophils. J. Cell Biol. 123, 895–907 (1993).
Hang, L. et al. Macrophage inflammatory protein-2 is required for neutrophil passage across the epithelial barrier of the infected urinary tract. J. Immunol. 162, 3037–3044 (1999).
Ghosh, S., May, M.J. & Kopp, E.B. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16, 225–260 (1998).
May, M.J. & Ghosh, S. Signal transduction through NF-κB. Immunol. Today 19, 80–88 (1998).
Schesser, K. et al. The YopJ locus is required for Yersinia-mediated inhibition of NF-κB activation and cytokine expression: YopJ contains a eukaryotic SH2-like domain that is essential for its repressive activity. Mol. Microbiol. 28, 1067–1079 (1998).
Cheng, Q. et al. NF-κB subunit-specific regulation of the IκBα promoter. J. Biol. Chem. 269, 13551–13557 (1994).
Chiao, P.J., Miyamoto, S. & Verma, I.M. Autoregulation of IκBα activity. Proc. Natl. Acad. Sci. USA 91, 28–32 (1994).
Haller, D. et al. TGFβ-1 inhibits non-pathogenic Gram negative bacteria-induced NF-κB recruitment to the IL-6 gene promoter in intestinal epithelial cells through modulation of histone acetylation. J. Biol. Chem. 278, 23851–23860 (2003).
Chen, L-f., Fischle, W., Verdin, E. & Greene, W.C. Duration of NF-κB action regulated by reversible acetylation. Science 293, 1653–1657 (2001).
Huang, T.T., Kudo, N., Yoshida, M. & Miyamoto, S. A nuclear export signal in the N-terminal regulatory domain of IκBα controls cytoplasmic localisation of inactive NF-κB/IκBα complexes. Proc. Natl. Acad. Sci. USA 97, 1014–1019 (2000).
Kudo, H. et al. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc. Natl. Acad. Sci. USA 96, 9112–9117 (1999).
Su, C.G. et al. A novel therapy for colitis utilizing PPAR-γ ligands to inhibit the epithelial inflammatory response. J. Clin. Invest. 104, 383–389 (1999).
Nakajima, A. et al. Endogenous PPARγ mediates anti-inflammatory activity in murine ischemia-reperfusion injury. Gastroenterol. 120, 460–469 (2001).
Wang, N. et al. Constitutive activation of peroxisome proliferator-activated receptor-γ suppresses pro-inflammatory adhesion molecules in human vascular endothelial cells. J. Biol. Chem. 277, 34176–34181 (2002).
Katayama, K. et al. A novel PPARγ gene therapy to control inflammation associated with inflammatory bowel disease in a murine model. Gastroenterol. 124, 1315–1324 (2003).
Chawla, K. et al. PPAR-γ dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat. Med. 7, 48–52 (2001).
Rossi, A. et al. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IκB kinase. Nature 403, 103–108 (2000).
Straus, D.S. et al. 15-Deoxy-Δ12,14-prostaglandin J2 inhibits multiple steps in the NF-κB signaling pathway. PNAS, 97, 4844–4849 (2000).
Bunn, C.F. et al. Nucleocytoplasmic shuttling of the thyroid hormone receptor α. Mol. Endocrinol. 15, 512–533 (2001).
Ricote, M., Huang, J.T., Welch, J.S. & Glass, C.K. The peroxisome proliferator-activated receptorγ (PPARγ) as a regulator of monocyte/macrophage function. J. Leukoc. Biol. 66, 733–739 (1999).
Chung, S.W. et al. Oxidized low density lipoprotein inhibits interleukin-12 production in lipopolysaccharide-activated mouse macrophages via direct interactions between peroxisome proliferator-activated receptor-γ and nuclear factor-κB. J. Biol. Chem. 275, 32681–32687 (2000).
Gurnell, M. et al. A dominant-negative peroxisome proliferator-activated receptor γ (PPARγ) mutant is a constitutive repressor and inhibits PPARγ-mediated adipogenesis. J. Biol. Chem. 275, 5754–5759 (2000).
Suzawa et al. Cytokines suppress adipogenesis and PPARγ function through the TAK1/TAB1/NIK cascade. Nature Cell Biol. 5, 224–230 (2003).
Schmid, J.A. et al. Dynamics of NF-κB and IκBα studied with green fluorescent protein (GFP) fusion proteins. Investigation of GFP-p65 binding to DNA by fluorescence resonance energy transfer. J. Biol. Chem. 275, 17035–17042 (2000).
Ghosh, S. & Karin, M. Missing pieces in the NF-κB puzzle. Cell 109, S81–S96 (2002).
Tam, W.F. & Sen, R. IκB family members function by different mechanisms. J. Biol. Chem. 276, 7701–7704 (2001).
Elbashir, S.M. et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411, 494–498 (2001).
Sui, G. et al. A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc. Natl. Acad. Sci. USA 99, 5515–5520 (2002).
Gewirtz, A.T., Navas, T.A., Lyons, S., Godowski, P.J. & Madara, J.L. Bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 167, 1882–1885 (2001).
De Winter, H. et al. Regulation of mucosal immune responses by interleukin 10 produced by intestinal epithelial cells in mice. Gastroenterol. 122, 1829–1841 (2002).
Di Leo, V., Yang, P.C., Berin, M.C. & Perdue, M.H. Factors regulating the effect of IL-4 on intestinal epithelial barrier function. Int. Arch. Allergy Immunol. 129, 219–227 (2002).
Xu, J. et al. A genomic view of the human-Bacteroides thetaiotaomicron symbosis. Science 299, 2074–2076 (2003).
Parkos, C.A., Delp, C., Arnaout, M.A. & Madara, J.L. Neutrophil migration across a cultured intestinal epithelium. Dependence on a CD11b/CD18-mediated event and enhanced efficiency in physiological direction. J. Clin. Invest. 88, 1605–1612 (1991).
Acknowledgements
We thank E.T. Logan, K.E Garden, D.J. Fraser-Pitt, D.L. Wilson and J.C. Martin for technical support. We also thank V.K. Chatterjee (Addenbrooke's Hospital, Cambridge, UK) and J.A. Schmid (University of Vienna, Austria) for providing the PPAR-γ and YFP-RelA clones for this work. Supported by SEERAD (Scottish Executive for Environmental and Rural Affairs Department).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Kelly, D., Campbell, J., King, T. et al. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-γ and RelA. Nat Immunol 5, 104–112 (2004). https://doi.org/10.1038/ni1018
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1018