Abstract
Smoking-related emphysema is a chronic inflammatory disease driven by the TH17 subset of helper T cells through molecular mechanisms that remain obscure. Here we explored the role of the microRNA miR-22 in emphysema. We found that miR-22 was upregulated in lung myeloid dendritic cells (mDCs) of smokers with emphysema and antigen-presenting cells (APCs) of mice exposed to smoke or nanoparticulate carbon black (nCB) through a mechanism that involved the transcription factor NF-κB. Mice deficient in miR-22, but not wild-type mice, showed attenuated TH17 responses and failed to develop emphysema after exposure to smoke or nCB. We further found that miR-22 controlled the activation of APCs and TH17 responses through the activation of AP-1 transcription factor complexes and the histone deacetylase HDAC4. Thus, miR-22 is a critical regulator of both emphysema and TH17 responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Accession codes
References
Hoyert, D.L. & Xu, J.Q. Deaths: preliminary data for 2011. Natl. Vital Stat. Rep. 61, 51–65 (2012).
Wilson, D.O. et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am. J. Respir. Crit. Care Med. 178, 738–744 (2008).
Hart, J.E., Eisen, E.A. & Laden, F. Occupational diesel exhaust exposure as a risk factor for chronic obstructive pulmonary disease. Curr. Opin. Pulm. Med. 18, 151–154 (2012).
Xu, C. et al. Autoreactive T cells in human smokers is predictive of clinical outcome. Front. Immunol. 3, 267 (2012).
Grumelli, S. et al. An immune basis for lung parenchymal destruction in chronic obstructive pulmonary disease and emphysema. PLoS Med. 1, 75–83 (2004).
Shan, M. et al. Lung myeloid dendritic cells coordinately induce TH1 and TH17 responses in human emphysema. Sci. Transl. Med. 1, 28 (2009).
Shan, M. et al. Agonistic induction of PPAR-γ reverses cigarette smoke induced emphysema. J. Clin. Invest. 124, 1371–1381 (2014).
Shan, M. et al. Cigarette smoke induction of osteopontin (SPP1) mediates TH17 inflammation in human and experimental emphysema. Sci. Transl. Med. 4, 18 (2012).
O'Connell, R.M., Rao, D.S., Chaudhuri, A.A. & Baltimore, D. Physiological and pathological roles for microRNAs in the immune system. Nat. Rev. Immunol. 10, 111–122 (2010).
Polikepahad, S. et al. Proinflammatory role for let-7 microRNAS in experimental asthma. J. Biol. Chem. 285, 30139–30149 (2010).
You, R. et al. Nanoparticulate carbon black in cigarette smoke induce DNA cleavage and Th17-mediated emphysema. eLife (in the press).
Rincon, M. & Irvin, C.G. Role of IL-6 in asthma and other inflammatory pulmonary diseases. Int. J. Biol. Sci. 8, 1281–1290 (2012).
Rodriguez, A., Griffiths-Jones, S., Ashurst, J.L. & Bradley, A. Identification of mammalian microRNA host genes and transcription units. Genome Res. 14, 1902–1910 (2004).
Hou, Y., Moreau, F. & Chadee, K. PPARγ is an E3 ligase that induces the degradation of NF-κB/p65. Nat. Commun. 3, 1300 (2012).
Shan, M. et al. Agonistic induction of PPARγ reverses cigarette smoke-induced emphysema. J. Clin. Invest. 124, 1371–1381 (2014).
Greenlee, K.J., Werb, Z. & Kheradmand, F. Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted. Physiol. Rev. 87, 69–98 (2007).
O'Sullivan, B.J. & Thomas, R. CD40 ligation conditions dendritic cell antigen-presenting function through sustained activation of NF-kB. J. Immunol. 168, 5491–5498 (2002).
Mann, J., Oakley, F., Johnson, P.W. & Mann, D.A. CD40 induces interleukin-6 gene transcription in dendritic cells: regulation by TRAF2, AP-1, NF-κB, AND CBF1. J. Biol. Chem. 277, 17125–17138 (2002).
Oeckinghaus, A., Hayden, M.S. & Ghosh, S. Crosstalk in NF-κB signaling pathways. Nat. Immunol. 12, 695–708 (2011).
Huang, Z.P. et al. MicroRNA-22 regulates cardiac hypertrophy and remodeling in response to stress. Circ. Res. 112, 1234–1243 (2013).
Jovicic, A., Zaldivar Jolissaint, J.F., Moser, R., Silva Santos Mde, F. & Luthi-Carter, R. MicroRNA-22 (miR-22) overexpression is neuroprotective via general anti-apoptotic effects and may also target specific Huntington's disease-related mechanisms. PLoS ONE 8, e54222 (2013).
Zhang, J. et al. microRNA-22, downregulated in hepatocellular carcinoma and correlated with prognosis, suppresses cell proliferation and tumourigenicity. Br. J. Cancer 103, 1215–1220 (2010).
de Ruijter, A.J., van Gennip, A.H., Caron, H.N., Kemp, S. & van Kuilenburg, A.B. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem. J. 370, 737–749 (2003).
Parra, M. Class IIa HDACs - new insights into their functions in physiology and pathology. FEBS J. 282, 1736–1744 (2015).
Luan, B. et al. Leptin-mediated increases in catecholamine signaling reduce adipose tissue inflammation via activation of macrophage HDAC4. Cell Metab. 19, 1058–1065 (2014).
Gordon, J.W. et al. Protein kinase A-regulated assembly of a MEF2-HDAC4 repressor complex controls c-Jun expression in vascular smooth muscle cells. J. Biol. Chem. 284, 19027–19042 (2009).
Wang, A.H. et al. HDAC4, a human histone deacetylase related to yeast HDA1, is a transcriptional corepressor. Mol. Cell. Biol. 19, 7816–7827 (1999).
Gurha, P. et al. Targeted deletion of microRNA-22 promotes stress-induced cardiac dilation and contractile dysfunction. Circulation 125, 2751–2761 (2012).
Gurha, P. et al. microRNA-22 promotes heart failure through coordinate suppression of PPAR/ERR-nuclear hormone receptor transcription. PLoS ONE 8, e75882 (2013).
Huang, S. et al. Upregulation of miR-22 promotes osteogenic differentiation and inhibits adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells by repressing HDAC6 protein expression. Stem Cells Dev. 21, 2531–2540 (2012).
Tang, Y. et al. microRNA-22 acts as a metastasis suppressor by targeting metadherin in gastric cancer. Mol. Med. Rep. 11, 454–460 (2015).
Xu, Q.F. et al. MiR-22 is frequently downregulated in medulloblastomas and inhibits cell proliferation via the novel target PAPST1. Brain Pathol. 24, 568–583 (2014).
Song, S.J. et al. The oncogenic microRNA miR-22 targets the TET2 tumor suppressor to promote hematopoietic stem cell self-renewal and transformation. Cell Stem Cell 13, 87–101 (2013).
Keatings, V.M., Jatakanon, A., Worsdell, Y.M. & Barnes, P.J. Effects of inhaled and oral glucocorticoids on inflammatory indices in asthma and COPD. Am. J. Respir. Crit. Care Med. 155, 542–548 (1997).
Mizuno, S. et al. Inhibition of histone deacetylase causes emphysema. Am. J. Physiol. Lung Cell. Mol. Physiol. 300, L402–L413 (2011).
Lemercier, C. et al. Class II histone deacetylases are directly recruited by BCL6 transcriptional repressor. J. Biol. Chem. 277, 22045–22052 (2002).
Sandhu, S.K. et al. miR-155 targets histone deacetylase 4 (HDAC4) and impairs transcriptional activity of B-cell lymphoma 6 (BCL6) in the Emu-miR-155 transgenic mouse model. Proc. Natl. Acad. Sci. USA 109, 20047–20052 (2012).
Han, S. et al. Recruitment of histone deacetylase 4 by transcription factors represses interleukin-5 transcription. Biochem. J. 400, 439–448 (2006).
Yang, Q. et al. Cross talk between histone deacetylase 4 and STAT6 in the transcriptional regulation of arginase 1 during mouse dendritic cell differentiation. Mol. Cell. Biol. 35, 63–75 (2015).
Liu, W. et al. AP-1 activated by toll-like receptors regulates expression of IL-23 p19. J. Biol. Chem. 284, 24006–24016 (2009).
Wang, X. et al. Early secreted antigenic target of 6-kDa protein of Mycobacterium tuberculosis primes dendritic cells to stimulate Th17 and inhibit Th1 immune responses. J. Immunol. 189, 3092–3103 (2012).
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006).
Ivanov, I.I. et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006).
Kudo, M. et al. IL-17A produced by αβ T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat. Med. 18, 547–554 (2012).
Röhn, T.A. et al. Vaccination against IL-17 suppresses autoimmune arthritis and encephalomyelitis. Eur. J. Immunol. 36, 2857–2867 (2006).
Elson, C.O. et al. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology 132, 2359–2370 (2007).
Hirota, K. et al. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J. Exp. Med. 204, 41–47 (2007).
Majdzadeh, N. et al. HDAC4 inhibits cell-cycle progression and protects neurons from cell death. Dev. Neurobiol. 68, 1076–1092 (2008).
Pauwels, R.A. et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am. J. Respir. Crit. Care Med. 163, 1256–1276 (2001).
Corry, D.B. et al. Interleukin 4, but not interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J. Exp. Med. 183, 109–117 (1996).
Lee, S.H. et al. Differential requirement for CD18 in T-helper effector homing. Nat. Med. 9, 1281–1286 (2003).
Corry, D.B. et al. Decreased allergic lung inflammatory cell egression and increased susceptibility to asphyxiation in MMP2-deficiency. Nat. Immunol. 3, 347–353 (2002).
Acknowledgements
We thank J. Levitt (Baylor College of Medicine) for CD11c-Cre mice; E. Olson (UT Southwestern Medical Center) for Hdac4fl mice; and W. Decker, J. Sederstrom, Y. Qian and L.-Z. Song for technical assistance. Supported by the US National Institutes of Health (R01HL117181 to F.K.; R01HL110883 to F.K. and D.B.C.; and AI036211, CA125123 and RR024574 to the Cytometry and Cell Sorting Core at Baylor College of Medicine) and the US Veterans Administration Office of Research and Development (1I01BX002221 to D.B.C.).
Author information
Authors and Affiliations
Contributions
W.L. and D.B.C. conceptualized the project and all studies and wrote the manuscript with assistance from all co-authors; W.L. designed and performed all experiments, with R.Y., X.Y. and T.Y. contributing to selected mouse experiments and micro-CT analysis; E.L.G.S., D.C.M., W.K.A.S. and J.M.T. prepared nCB; A.R. provided miR-22 deficient mice; and F.K. and D.B.C. provided grant support.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
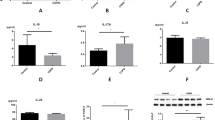
Supplementary Figure 1 miR-22 is required for airway MMP activities but not for the development of γδ+ T cells in emphysema.
(a) Gelatin zymogram depicting matrix metalloproteinase (MMP) 2 and MMP9 activities in bronchoalveolar lavage fluid (BALF) of wild type (WT; lanes 3-5) and Mir22–/– mice (lanes 6-9) previously challenged with nCB. Lanes 1 and 2 are BALF from naïve wild type mice. (b) Total lung IL-17A+ γδ+ T cells were quantified by flow cytometry.
Supplementary Figure 2 Mir22–/– mice develop reduced TH17 responses upon sensitization with ovalbumin.
Both WT and Mir22–/– mice were intraperitoneally injected with 25µg ovalbumin precipitated in alum weekly for three consecutive weeks. Total splenocytes were harvested at the end of the fifth week. (a) IFN-γ, (b) IL-4 and (c) IL-17A positive cells with ovalbumin (OVA; 0.5 mg/ml overnight) and without (Media) were quantified by ELISpot. *: p<0.05, Kruskal Wallis test. n=4.
Supplementary Figure 3 nCB-induced production of pro-inflammatory cytokines and chemokines from whole lung is in part miR-22 dependent.
WT and Mir22–/– mice were challenged intranasally with nCB over one month. (a-d) Lungs were then collected and the concentrations of the indicated cytokines and chemokines from lung homogenate fluids were determined by Bio-plex. (e-g) Lung CD11c+ cells were isolated and cultured ex vivo overnight. Concentrations of the indicated supernatant chemokines were determined by Bio-plex. *: p<0.05; **: p<0.01, ***: p<0.001, Kruskal Wallis test. n=4-5.
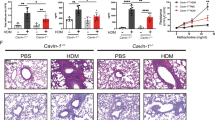
Supplementary Figure 4 Smoke-exposed wild-type lung APCs are sufficient to induce emphysema in mice with and without miR-22.
Lung CD11c+ APC from four month cigarette SMK or air exposed WT mice were isolated and adaptively transferred to WT and Mir22–/– recipients. (a) Micro-CT quantification of recipient mouse lung volume after 3 months. (b) Total macrophages in BALF. (c) Relative abundance of lung IL-17A+ TH17 cells as assessed by flow cytometry. *: p<0.05; **: p<0.01, Kruskal Wallis test. n=3-4 as indicated in (A).
Supplementary Figure 5 PPAR-γ regulates miR-22 expression in lung APCs.
(a) MiR-22 expression in CD11c+ lung APCs from eight month old Ppargflox and PpargCD11c mice. (b) MiR-22 expression in CD11c+ lung APCs from air or cigarette smoke (SMK) exposed mice with or without intranasally challenge of ciglitazone (Cig). *: p<0.05; unpaired t-test in (a) one-way ANOVA test in (b). (n=3)
Supplementary Figure 6 miR-22 is required for pro-inflammatory gene expression in nCB-exposed lung APCs.
Lung CD11c+ cells from WT and Mir22–/– mice exposed to PBS or nCB were isolated. Expression of indicated genes was determined by quantitative PCR. *, p<0.05; **, p<0.01; ***, p<0.001, One-way ANOVA test. (n=3)
Supplementary Figure 7 miR-22 deficient APCs induce a TH17 cytokine in vitro with supplementation of IL-6.
MiR-22 sufficient (Mir22flox) and deficient BMDCs (Mir22CD11c) were primed with or without 1000ng nCB and co-cultured with WT naïve CD4+ T cells for 3 days and secreted IL-17A was measured by ELISA (n=4-5). 10ng/ml IL-6 supplement was added as indicated. *: p<0.05; Mann-Whitney test (n=4-5).
Supplementary Figure 8 Silencing miR-22 alters pro- and anti-inflammatory gene expression in APCs.
Mice were sacrificed at the 5 month point in Figure (8a). (a) MiR-22 expression level in lung CD11c+ cells. IL-6 (b) and IL-17A (c) concentration in lung homogenates as assessed by Bioplex. (d-f) Indicated gene expression in isolated lung CD11c+ cells determined by RT-qPCR. *, p<0.05; **, p<0.01; ***, p<0.001, Kruskal Wallis test (n=4-6). Data in are from one of two comparable experiments.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 (PDF 1105 kb)
Rights and permissions
About this article
Cite this article
Lu, W., You, R., Yuan, X. et al. The microRNA miR-22 inhibits the histone deacetylase HDAC4 to promote TH17 cell–dependent emphysema. Nat Immunol 16, 1185–1194 (2015). https://doi.org/10.1038/ni.3292
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3292
This article is cited by
-
Insights into the toxicological effects of nanomaterials on atherosclerosis: mechanisms involved and influence factors
Journal of Nanobiotechnology (2023)
-
The phosphatidylinositol-transfer protein Nir3 promotes PI(4,5)P2 replenishment in response to TCR signaling during T cell development and survival
Nature Immunology (2023)
-
MicroRNA-22 represses glioma development via activation of macrophage-mediated innate and adaptive immune responses
Oncogene (2022)
-
Inhibition of MicroRNA-214 Alleviates Lung Injury and Inflammation via Increasing FGFR1 Expression in Ventilator-Induced Lung Injury
Lung (2021)
-
Genetic deletion of microRNA-22 blunts the inflammatory transcriptional response to status epilepticus and exacerbates epilepsy in mice
Molecular Brain (2020)