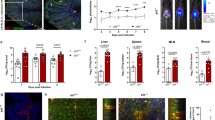
Abstract
The epithelium is the main entry point for many viruses, but the processes that protect barrier surfaces against viral infections are incompletely understood. Here we identified interleukin 22 (IL-22) produced by innate lymphoid cell group 3 (ILC3) as an amplifier of signaling via interferon-λ (IFN-λ), a synergism needed to curtail the replication of rotavirus, the leading cause of childhood gastroenteritis. Cooperation between the receptor for IL-22 and the receptor for IFN-λ, both of which were 'preferentially' expressed by intestinal epithelial cells (IECs), was required for optimal activation of the transcription factor STAT1 and expression of interferon-stimulated genes (ISGs). These data suggested that epithelial cells are protected against viral replication by co-option of two evolutionarily related cytokine networks. These data may inform the design of novel immunotherapy for viral infections that are sensitive to interferons.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Moon, C. & Stappenbeck, T.S. Viral interactions with the host and microbiota in the intestine. Curr. Opin. Immunol. 24, 405–410 (2012).
Duerkop, B.A. & Hooper, L.V. Resident viruses and their interactions with the immune system. Nat. Immunol. 14, 654–659 (2013).
Katze, M.G., He, Y. & Gale, M. Jr. Viruses and interferon: a fight for supremacy. Nat. Rev. Immunol. 2, 675–687 (2002).
Durbin, R.K., Kotenko, S.V. & Durbin, J.E. Interferon induction and function at the mucosal surface. Immunol. Rev. 255, 25–39 (2013).
Kotenko, S.V. et al. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat. Immunol. 4, 69–77 (2003).
Sheppard, P. et al. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat. Immunol. 4, 63–68 (2003).
Dumoutier, L. et al. Role of the interleukin (IL)-28 receptor tyrosine residues for antiviral and antiproliferative activity of IL-29/interferon-λ1: similarities with type I interferon signaling. J. Biol. Chem. 279, 32269–32274 (2004).
Ank, N. et al. Lambda interferon (IFN-λ), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J. Virol. 80, 4501–4509 (2006).
Doyle, S.E. et al. Interleukin-29 uses a type 1 interferon-like program to promote antiviral responses in human hepatocytes. Hepatology 44, 896–906 (2006).
Sadler, A.J. & Williams, B.R. Interferon-inducible antiviral effectors. Nat. Rev. Immunol. 8, 559–568 (2008).
Kotenko, S.V. IFN-lambdas. Curr. Opin. Immunol. 23, 583–590 (2011).
Wolk, K. et al. IL-22 increases the innate immunity of tissues. Immunity 21, 241–254 (2004).
Sommereyns, C., Paul, S., Staeheli, P. & Michiels, T. IFN-lambda (IFN-λ) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog. 4, e1000017 (2008).
Rutz, S. & Ouyang, W. Regulation of interleukin-10 and interleukin-22 expression in T helper cells. Curr. Opin. Immunol. 23, 605–612 (2011).
Zheng, Y. et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 14, 282–289 (2008).
Pickert, G. et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J. Exp. Med. 206, 1465–1472 (2009).
Ramig, R.F. Pathogenesis of intestinal and systemic rotavirus infection. J. Virol. 78, 10213–10220 (2004).
Sen, A., Rott, L., Phan, N., Mukherjee, G. & Greenberg, H.B. Rotavirus NSP1 protein inhibits interferon-mediated STAT1 activation. J. Virol. 88, 41–53 (2014).
Holloway, G. & Coulson, B.S. Innate cellular responses to rotavirus infection. J. Gen. Virol. 94, 1151–1160 (2013).
Arnold, M.M., Sen, A., Greenberg, H.B. & Patton, J.T. The battle between rotavirus and its host for control of the interferon signaling pathway. PLoS Pathog. 9, e1003064 (2013).
Pott, J. et al. IFN-λ determines the intestinal epithelial antiviral host defense. Proc. Natl. Acad. Sci. USA 108, 7944–7949 (2011).
Feng, N. et al. Role of interferon in homologous and heterologous rotavirus infection in the intestines and extraintestinal organs of suckling mice. J. Virol. 82, 7578–7590 (2008).
Sen, A. et al. Innate immune response to homologous rotavirus infection in the small intestinal villous epithelium at single-cell resolution. Proc. Natl. Acad. Sci. USA 109, 20667–20672 (2012).
Kumar, P., Thakar, M.S., Ouyang, W. & Malarkannan, S. IL-22 from conventional NK cells is epithelial regenerative and inflammation protective during influenza infection. Mucosal Immunol. 6, 69–82 (2013).
Ubeda, C. et al. Familial transmission rather than defective innate immunity shapes the distinct intestinal microbiota of TLR-deficient mice. J. Exp. Med. 209, 1445–1456 (2012).
Zenewicz, L.A. et al. IL-22 deficiency alters colonic microbiota to be transmissible and colitogenic. J. Immunol. 190, 5306–5312 (2013).
Lauterbach, H. et al. Mouse CD8alpha+ DCs and human BDCA3+ DCs are major producers of IFN-lambda in response to poly IC. J. Exp. Med. 207, 2703–2717 (2010).
Yin, Z. et al. Type III IFNs are produced by and stimulate human plasmacytoid dendritic cells. J. Immunol. 189, 2735–2745 (2012).
Sanos, S.L. et al. RORγt and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat. Immunol. 10, 83–91 (2009).
Liang, S.C. et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203, 2271–2279 (2006).
Martin, B., Hirota, K., Cua, D.J., Stockinger, B. & Veldhoen, M. Interleukin-17-producing γδ T cells selectively expand in response to pathogen products and environmental signals. Immunity 31, 321–330 (2009).
Zindl, C.L. et al. IL-22-producing neutrophils contribute to antimicrobial defense and restitution of colonic epithelial integrity during colitis. Proc. Natl. Acad. Sci. USA 110, 12768–12773 (2013).
Klose, C.S. et al. A T-bet gradient controls the fate and function of CCR6-RORγt+ innate lymphoid cells. Nature 494, 261–265 (2013).
Kiss, E.A. et al. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science 334, 1561–1565 (2011).
Eberl, G. et al. An essential function for the nuclear receptor RORγ(t) in the generation of fetal lymphoid tissue inducer cells. Nat. Immunol. 5, 64–73 (2004).
Franco, M.A. & Greenberg, H.B. Immunity to rotavirus infection in mice. J. Infect. Dis. 179 (suppl. 3), S466–S469 (1999).
Kinnebrew, M.A. et al. Interleukin 23 production by intestinal CD103+CD11b+ dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity 36, 276–287 (2012).
Reynders, A. et al. Identity, regulation and in vivo function of gut NKp46+RORγt+ and NKp46+RORγt− lymphoid cells. EMBO J. 30, 2934–2947 (2011).
Schoggins, J.W. & Rice, C.M. Interferon-stimulated genes and their antiviral effector functions. Curr. Opin. Virol. 1, 519–525 (2011).
Bachmann, M., Ulziibat, S., Hardle, L., Pfeilschifter, J. & Muhl, H. IFNα converts IL-22 into a cytokine efficiently activating STAT1 and its downstream targets. Biochem. Pharmacol. 85, 396–403 (2013).
Lejeune, D. et al. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. Pathways that are shared with and distinct from IL-10. J. Biol. Chem. 277, 33676–33682 (2002).
Dai, X. et al. SOCS1-negative feedback of STAT1 activation is a key pathway in the dsRNA-induced innate immune response of human keratinocytes. J. Invest. Dermatol. 126, 1574–1581 (2006).
Angel, J., Franco, M.A., Greenberg, H.B. & Bass, D. Lack of a role for type I and type II interferons in the resolution of rotavirus-induced diarrhea and infection in mice. J. Interferon Cytokine Res. 19, 655–659 (1999).
Nice, T.J. et al. Interferon-λ cures persistent murine norovirus infection in the absence of adaptive immunity. Science 347, 269–273 (2015).
Baldridge, M.T. et al. Commensal microbes and interferon-λ determine persistence of enteric murine norovirus infection. Science 347, 266–269 (2015).
Muñoz, M. et al. Interleukin-22 induces interleukin-18 expression from epithelial cells during intestinal infection. Immunity 42, 321–331 (2015).
Aujla, S.J. et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 14, 275–281 (2008).
Dudakov, J.A. et al. Interleukin-22 drives endogenous thymic regeneration in mice. Science 336, 91–95 (2012).
Hanash, A.M. et al. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity 37, 339–350 (2012).
Zhang, B. et al. Viral infection. Prevention and cure of rotavirus infection via TLR5/NLRC4-mediated production of IL-22 and IL-18. Science 346, 861–865 (2014).
Mombaerts, P. et al. Mutations in T-cell antigen receptor genes α and β block thymocyte development at different stages. Nature 360, 225–231 (1992).
Itohara, S. et al. T cell receptor δ gene mutant mice: independent generation of αβ T cells and programmed rearrangements of γδ TCR genes. Cell 72, 337–348 (1993).
Alonzi, T. et al. Essential role of STAT3 in the control of the acute-phase response as revealed by inducible gene inactivation [correction of activation] in the liver. Mol. Cell. Biol. 21, 1621–1632 (2001).
el Marjou, F. et al. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 39, 186–193 (2004).
Kreymborg, K. et al. IL-22 is expressed by Th17 cells in an IL-23-dependent fashion, but not required for the development of autoimmune encephalomyelitis. J. Immunol. 179, 8098–8104 (2007).
Ank, N. et al. An important role for type III interferon (IFN-λ/IL-28) in TLR-induced antiviral activity. J. Immunol. 180, 2474–2485 (2008).
Durbin, J.E., Hackenmiller, R., Simon, M.C. & Levy, D.E. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell 84, 443–450 (1996).
Eberl, G. & Littman, D.R. Thymic origin of intestinal αβ T cells revealed by fate mapping of RORγt+ cells. Science 305, 248–251 (2004).
Glaccum, M.B. et al. Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. J. Immunol. 159, 3364–3371 (1997).
Ghilardi, N. et al. Compromised humoral and delayed-type hypersensitivity responses in IL-23-deficient mice. J. Immunol. 172, 2827–2833 (2004).
Sanos, S.L. & Diefenbach, A. Isolation of NK cells and NK-like cells from the intestinal lamina propria. Methods Mol. Biol. 612, 505–517 (2010).
Yang, I. et al. Intestinal microbiota composition of interleukin-10 deficient C57BL/6J mice and susceptibility to Helicobacter hepaticus-induced colitis. PLoS ONE 8, e70783 (2013).
Edgar, R.C. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10, 996–998 (2013).
Wang, Q., Garrity, G.M., Tiedje, J.M. & Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73, 5261–5267 (2007).
Cole, J.R. et al. Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res. 42, D633–D642 (2014).
Yarza, P. et al. The All-Species Living Tree project: a 16S rRNA-based phylogenetic tree of all sequenced type strains. Syst. Appl. Microbiol. 31, 241–250 (2008).
Schloss, P.D. et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541 (2009).
Oksanen, J. et al. vegan: Community Ecology Package http://CRAN.R-project.org/package=vegan (2013).
Livak, K.J. & Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCT) method. Methods 25, 402–408 (2001).
Acknowledgements
We thank V. Sexl (University of Veterinary Medicine Vienna) for mice with a Stat3fl allele (provided with permission from V. Poli, Universita di Torino); M. Stemmler (Max-Planck-Institute of Immunobiology & Epigenetics, Freiburg) for Vil1-Cre mice (provided with permission from S. Robine. Institute Curie, Paris); C. Johner (Max-Planck-Institute of Immunobiology & Epigenetics, Freiburg) for Rag2−/− and Rag2−/−Il2rg−/− mice; D. Littman (Skirball Institute of Biomolecular Medicine) for Rorc-CreTG mice; N. Ghilardi (Genentech) for Il23a−/− mice; M. Hornef (Hannover Medical School) for the rat IEC line IEC6 and the mouse rotavirus strain EDIM and for discussions; S. Koike (Tokyo Metropolitan Institute of Medical Science) for poliovirus type I Mahoney strain; G. Häcker for support; members of the Diefenbach laboratory for discussions; A. Triantafyllopoulou for critical reading of the manuscript; K. Oberle, S. Woltemate and Z. Fiebig for technical assistance; and M. Follo, K. Geiger and J. Bodinek-Wersing for cell sorting. Supported by ERC (311377 to A.D.), Deutsche Forschungsgemeinschaft (Priority Program 1656 “Intestinal Microbiota” DI764/3 to A.D. and SU133/9 to S.S.; GRK1104 to A.D. and F.G.; and SFB900/Z1 to S.S.) and the Excellence Initiative of the Deutsche Forschungsgemeinschaft and the German Science Council (Spemann Graduate School of Biology and Medicine (GSC-4 to T.M.); and the Cluster of Excellence “Inflammation at Interfaces” (Borstel-Kiel-Lübeck-Plön) EXC306 to C.H.).
Author information
Authors and Affiliations
Contributions
P.P.H. and T.M. performed most of the experiments with the help of V.S., N.N., F.G. and K.G.; I.Y. and S.S. performed the microbiota analysis; B.R., C.H., L.D. and J.-C.R. provided mice, reagents and input on the manuscript; P.S. and A.D. designed the study; P.P.H., T.M., P.S. and A.D. analyzed the data; and A.D. directed the study and wrote the manuscript (with contributions from P.P.H., T.M. and P.S.).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Gene-expression analysis during the first days of rotavirus infection.
a-i, Groups of wildtype suckling mice were infected with 7x102 ID50 rotavirus by oral inoculation. Transcript levels of the indicated genes were quantified by RT-qPCR from small intestinal tissue obtained at the specified time-points after infection. All RT-qPCR data are shown as fold (2-∆Ct) relative to Hprt. n ≥ 3. Mean ± SEM.
Supplementary Figure 2 Presence of a functional Mx1 gene has no effect on the control of rotavirus infection in suckling mice.
(a) Groups of suckling wildtype mice with a functional (Mx1+) or a non-functional (Mx1−) Mx1 allele were infected with rotavirus. Virus titers and transcript levels of the indicated genes were quantified by RT-qPCR from small intestinal tissue obtained at the indicated time-points after oral infection. n ≥ 3. Mean ± SEM. One-way ANOVA. NS, not significant (P > 0.05). (b) H&E stain of small intestinal tissue of suckling mice at day 4 after rotavirus infection to illustrate virus-mediated tissue damage. Circled areas: vacuolizations; arrowheads: epithelial erosions; arrows: villus disruptions. Scale bar, 100μm. (c, d) Adult (6-8 week old) mice of the indicated strains were orally infected with rotavirus. Rotavirus transcripts in small intestine at day 4 after infection were determined by RT-qPCR (c). Virus shedding via feces was quantified by ELISA on day 4 (d). Mean ± SEM. n ≥ 9, One-way ANOVA. NS, not significant (P > 0.05). (e-h) Groups of suckling mice of the indicated genotypes were infected with rotavirus and were injected with BrdU at day 3 following infection. BrdU incorporation by IECs was assessed. (e, f) Numbers of BrdU-positive IECs per crypt where quantified 2 hours after BrdU injection. (g, h) BrdU incorporation by IECs at 24 hours after BrdU pulse starting at day three after infection. Relative distance of BrdU-positive IECs from crypt bottom to villus tip as percentage of villus length (h). Data are representative of two independent experiments. All RT-qPCR data are shown as fold (2-∆Ct) relative to Hprt. (e-h) n ≥ 3. Mean ± SEM; Student’s t test. One-way ANOVA. *P < 0.05; **P < 0.01; ***P < 0.001. NS, not significant (P > 0.05). Scale bars, 50 μm.
Supplementary Figure 3 Composition of microbial communities in Il22−/− and Ifnlr1−/− mice.
(a-f) Rarefaction curves of the 16S rDNA library and PCoA plots of microbiota compositions. Rarefaction curves of the 16S rDNA library OTU dataset. Adult mice (a), individual samples. Colour coded by genotype and litter. Suckling mice (d): Average values of litters of one genotype (solid lines), with standard deviations (dotted lines). PCoA plot of the microbiota composition of adult mice (b, c) of two different genotypes from two different litters, based on Jaccard index distances. PC 1 explains 23.1% of the variation; PC 2, 18.2%, and PC 3, 17.7%. One litter marked by squares (n = 4), the other by triangles (n = 6); Il22−/− mice are marked in blue (n = 5), WT mice in violet (n = 5). PCoA plot of the microbiota composition of suckling mice (e, f) of different genotypes, based on Jaccard index distances. One litter per genotype. PC 1 explains 20.9 % of the variation; PC 2, 18.7 %, and PC 3, 12.1%. Suckling Il22−/− mice are marked in green (n = 4), B6.A2G-Mx1-Il22−/− (MX_IL22_KO) sucklings in dark blue (n = 5), B6.A2G-Mx1-Ifnlr1−/− (MX_lamdaR_KO) sucklings in blue-green (n = 5), and B6.A2G-Mx1 (MX_WT) sucklings in pink (n = 5).
Supplementary Figure 4 IFN-λ is produced by intestinal epithelial cells.
Groups of suckling and adult wildtype mice were infected with rotavirus. Control groups received PBS. (a) Ifnl2/3 expression in small intestinal epithelial cells (IEC, black columns) and lamina propria leukocytes (LPL, white columns) isolated from adult mice at day 4 following infection was determined by RT-qPCR. (b) Rotavirus load in purified cells of the small intestine from suckling mice at day 4 after infection was determined by RT-qPCR. Purity of isolated cell fractions confirmed by expression of leukocyte marker Ptprc (encoding CD45) (c) or epithelial marker Cdh1 (encoding E-cadherin) (d). (e-h) Cells isolated from small intestinal tissues of rotavirus infected suckling mice at day 4 following rotavirus infection were stained for CD45 (hematopoietic cells) and EpCAM (IECs). The dot plot represents the gating strategy to isolate highly purified hematopoietic cells (CD45+ EpCAM-) or IECs (CD45- EpCAM+) (see Fig. 2c). (f-h) ISG expression in highly purified IECs (CD45− EpCAM+) and hematopoietic cells (CD45+ EpCAM−) was analyzed by RT-qPCR using RNA obtained from day 1 infected suckling mice. All RT-qPCR data are shown as fold (2-∆Ct) relative to Hprt. (a-h) Mean ± SEM. (a-d) n ≥ 6. (f-h) n ≥ 3. Data are representative of three (a-d) and two (f-h) independent experiments. (a, c, d, f-h) Two-way ANOVA. (b) Student’s t test. *P < 0.05; **P < 0.01; ***P < 0.001.
Supplementary Figure 5 Upregulation of IL-22 expression by CCR6+ RORγt+ ILC3 cells following rotavirus infection does not require IFN-λ signaling.
(a-e) Groups of suckling or adult mice of the indicated genotypes were orally infected with rotavirus and samples were collected at the indicated days following infection. Uninfected mice received PBS (mock) injections. (a) Il22 expression (RT-qPCR) in IECs and LPLs from suckling mice at day 1 following infection. (b-d) LPLs from the small intestine of suckling mice at day 1 following infection were stained with the indicated antibodies. Histograms (c) represent analysis of RORγt expression of gated IL-22− cells (grey) or IL-22+ (open) cells of all CD19− CD11b− cells (b). Contour plots (d) represent CCR6 and RORγt expression by IL-22− or IL-22+ cells of all CD19− CD11b− cells. (e) Rotavirus titers measured by ELISA on day 4 post infection in adult mice of the indicated genotypes. (f-i) Suckling mice were infected with rotavirus and expression of the indicated genes in small intestinal tissue was determined by RT-qPCR at day 1 following infection. Small intestinal sections were stained as indicated (i). Scale bars, 50 μm. All RT-qPCR data are shown as fold (2-∆Ct) relative to Hprt. (a-h) Mean ± SEM; n ≥ 5. Data are representative of three (a-d, f-i) and two (e) independent experiments (a, f-h) Two-way ANOVA. (e) One-way ANOVA. *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant (P > 0.05). ND, not detectable (or not able to be displayed on the scale here).
Supplementary Figure 6 IL-1α is required for rotavirus-induced expression of IL-22 by RORγt+ ILC3 cells.
Groups of suckling mice were orally infected with rotavirus. (a, b) Expression of Il1b and Il23a in IECs and LPLs from wildtype mice at day 1 following infection was analyzed by RT-qPCR. (c) Ifnl2/3 expression at day 1 following infection of the indicated mouse strains was analyzed by RT-qPCR using RNA from small intestinal tissue. (d-i) Twelve hours prior to infection, wildtype mice received intraperitoneal injections with 100 μg of the indicated neutralizing antibody. Il22 expression at day 1 following infection was analyzed by RT-qPCR using RNA from small intestinal tissue (d). Percentage (e) and absolute numbers (f) of IL-22+ cells among CD11b− CD19− CD3− RORγt+ lamina propria leukocytes at day 1 after infection. Rotavirus titers in small intestinal tissue determined by RT-qPCR (g). (h-i) Expression of IL-1-inducible genes by RT-qPCR. All RT-qPCR data are shown as fold (2-∆Ct) relative to Hprt. (a-i) Mean ± SEM. (a, b) n = 5. (c) n ≥ 3. (d-i) n ≥ 4. Data are representative of three (a-c) and two (e-i) independent experiments. (a-c, e-g) Two-way ANOVA. (d, h, i) One-way ANOVA. *P < 0.05; **P < 0.01; NS, not significant (P > 0.05). UI, uninfected mice. ND, not detectable (or not able to be displayed on the scale here).
Supplementary Figure 7 IL-22 and IFN-λ act synergisitcally for ISG induction.
Gene expression analysis by RT-qPCR of isolated small intestinal epithelial cells from rotavirus infected animals sacrificed at day 1 (a) or day 4 (b) after infection. The expression of 114 genes (ISGs, IL-22-regulated genes, STAT1 and/or STAT3 targets and genes involved in tissue repair) was analysed. Genes are divided as ISG and non-ISGs based on the Interferome online database (http://interferome.its.monash.edu.au) and organized according to the fold induction upon rotavirus infection in WT mice. Heat maps depict the ratio of gene expression as indicated. Thresholds of the color code are indicated at the bottom. (a, b) Left row indicates the ratio of gene expression between infected (I) vs. uninfected (UI) wildtype (WT) mice, middle row indicates the ratio of gene expression ratio between infected Il22−/− vs. infected WT mice and the right column indicates gene expression ratio between infected Ifnlr1−/− vs. infected WT mice. (c, d) Gene expression analysis by RT-qPCR in isolated IECs from rotavirus infected or mock infected animals. (e-l) The intestinal epithelial cell lines IEC6 (e-g) and Caco2 (i-l) were stimulated for 16 hours with the indicated amounts of mouse (for IEC6) or human (for Caco2) IFN-λ2 alone or with 100 ng/ml of mouse or human IL-22. Expression of the indicated ISGs was evaluated by RT-qPCR. All RT-qPCR data are shown as fold (2-∆Ct) relative to Hprt. (c-k) Mean ± SEM. (a, b) n ≥ 5. (c, d) n ≥ 4. (e-k) n = 4. Data are representative of two (a, b) or three (c-l) independent experiments. (e-l) Student’s t test. (c, d) Two-way ANOVA. *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant (P > 0.05). ND, not detectable (or not able to be displayed on the scale here).
Supplementary Figure 8 IL-22 and IFN-λ act together for the phosphorylation of STAT1.
(a-f) Specific deletion of STAT3 in intestinal epithelial cells. Analysis of the expression of Stat3 (a, b) and the indicated Stat1 (c) and Stat3 (d-f) target genes by RT-qPCR in IECs and LPLs isolated from adult wildtype and Stat3∆IEC mice. Stat3 transcripts were determined by using primer pairs outside (a) and inside (b) the deleted region. (g) Wildtype mice were subcutaneously injected with 1 μg of IFN-λ2 and/or 1 μg of IL-22 and sacrificed 1 hour post injection when STAT phosphorylation was assessed in small intestinal tissue. (h-i) Phospho-STAT analysis in intestinal epithelial cell lines IEC6 (h) and Caco2 (i) stimulated as indicated with 20 ng/ml of murine or human IFN-λ2 and 100 ng/ml of murine or human IL-22 for 30 min. For quantification, Image Studio Software for Odyssey® Fc System was used to image the blots and quantify the signal. Phospho-STAT values were normalized to the respective non-phosphorylated STATs. IFN-λ-stimulated sample signal was set to 100% for pSTAT1/STAT1 signal analysis and IL-22-stimulated sample signal was set to 100% in pSTAT3/STAT3 signal analysis. Data from three different experiments were pooled. (j) Groups of suckling mice from the indicated mouse strains were orally infected with rotavirus. Expression of the indicated genes in small intestinal tissue from suckling mice was determined by RT-qPCR at day 4 following infection. (k) Gene knockdown for the indicated STAT proteins was performed in IEC6 cells using siRNAs. Cells were stimulated with 100 ng/ml of IL-22 and/or IFN-λ2 for 16 hours. Gene expression was evaluated by RT-qPCR (upper graph) or Western blotting (below). All RT-qPCR data are shown as fold (2-∆Ct) relative to Hprt. Mean ± SEM. (a-f) n ≥ 3. (g-i) n = 4. (j) n ≥ 3. (k) n = 4. Data are representative of three (a-j) or four (k) independent experiments. (a-j) One-way ANOVA. (k) Student’s t test. *P < 0.05; **P < 0.01; ***P < 0.001. NS, not significant (P > 0.05). ND, not detectable (or not able to be displayed on the scale here).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Tables 1–4 (PDF 1124 kb)
Supplementary Data Set 1
OTU representation in microbiota samples (XLSX 39 kb)
Rights and permissions
About this article
Cite this article
Hernández, P., Mahlakõiv, T., Yang, I. et al. Interferon-λ and interleukin 22 act synergistically for the induction of interferon-stimulated genes and control of rotavirus infection. Nat Immunol 16, 698–707 (2015). https://doi.org/10.1038/ni.3180
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3180
This article is cited by
-
Disease pathogenesis and barrier functions regulated by group 3 innate lymphoid cells
Seminars in Immunopathology (2024)
-
Interleukin-22 facilitates the interferon-λ-mediated production of tripartite motif protein 25 to inhibit replication of duck viral hepatitis A virus type 1
Veterinary Research (2023)
-
PLZF restricts intestinal ILC3 function in gut defense
Cellular & Molecular Immunology (2023)
-
Microbiota-derived tryptophan metabolites in vascular inflammation and cardiovascular disease
Amino Acids (2022)
-
Possible association of rotavirus IgG with cytokine expression levels and dyslipidemia in rotavirus-infected type 1 diabetic children
Molecular Biology Reports (2022)