Abstract
The transcription factor BATF is required for the differentiation of interleukin 17 (IL-17)-producing helper T cells (TH17 cells) and follicular helper T cells (TFH cells). Here we identified a fundamental role for BATF in regulating the differentiation of effector of CD8+ T cells. BATF-deficient CD8+ T cells showed profound defects in effector population expansion and underwent proliferative and metabolic catastrophe early after encountering antigen. BATF, together with the transcription factors IRF4 and Jun proteins, bound to and promoted early expression of genes encoding lineage-specific transcription-factors (T-bet and Blimp-1) and cytokine receptors while paradoxically repressing genes encoding effector molecules (IFN-γ and granzyme B). Thus, BATF amplifies T cell antigen receptor (TCR)-dependent expression of transcription factors and augments the propagation of inflammatory signals but restrains the expression of genes encoding effector molecules. This checkpoint prevents irreversible commitment to an effector fate until a critical threshold of downstream transcriptional activity has been achieved.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Kaech, S.M., Wherry, E.J. & Ahmed, R. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2, 251–262 (2002).
Doering, T.A. et al. Network analysis reveals centrally connected genes and pathways involved in CD8+ T cell exhaustion versus memory. Immunity 37, 1130–1144 (2012).
Kaech, S.M. & Wherry, E.J. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity 27, 393–405 (2007).
van der Windt, G.J. & Pearce, E.L. Metabolic switching and fuel choice during T-cell differentiation and memory development. Immunol. Rev. 249, 27–42 (2012).
Rutishauser, R.L. & Kaech, S.M. Generating diversity: transcriptional regulation of effector and memory CD8 T-cell differentiation. Immunol. Rev. 235, 219–233 (2010).
Belz, G.T. & Kallies, A. Effector and memory CD8+ T cell differentiation: toward a molecular understanding of fate determination. Curr. Opin. Immunol. 22, 279–285 (2010).
Kaech, S.M. & Cui, W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 12, 749–761 (2012).
Pearce, E.L. et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science 302, 1041–1043 (2003).
Intlekofer, A.M. et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 6, 1236–1244 (2005).
Joshi, N.S. et al. Inflammation directs memory precursor and short-lived effector CD8+ T cell fates via the graded expression of T-bet transcription factor. Immunity 27, 281–295 (2007).
Rutishauser, R.L. et al. Transcriptional repressor Blimp-1 promotes CD8+ T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity 31, 296–308 (2009).
Kallies, A., Xin, A., Belz, G.T. & Nutt, S.L. Blimp-1 transcription factor is required for the differentiation of effector CD8+ T cells and memory responses. Immunity 31, 283–295 (2009).
Cannarile, M.A. et al. Transcriptional regulator Id2 mediates CD8+ T cell immunity. Nat. Immunol. 7, 1317–1325 (2006).
Schraml, B.U. et al. The AP-1 transcription factor Batf controls TH17 differentiation. Nature 460, 405–409 (2009).
Betz, B.C. et al. Batf coordinates multiple aspects of B and T cell function required for normal antibody responses. J. Exp. Med. 207, 933–942 (2010).
Ise, W. et al. The transcription factor BATF controls the global regulators of class-switch recombination in both B cells and T cells. Nat. Immunol. 12, 536–543 (2011).
Murphy, T.L., Tussiwand, R. & Murphy, K.M. Specificity through cooperation: BATF-IRF interactions control immune-regulatory networks. Nat. Rev. Immunol. 13, 499–509 (2013).
Grigoryan, G., Reinke, A.W. & Keating, A.E. Design of protein-interaction specificity gives selective bZIP-binding peptides. Nature 458, 859–864 (2009).
Quigley, M. et al. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nat. Med. 16, 1147–1151 (2010).
Glasmacher, E. et al. A genomic regulatory element that directs assembly and function of immune-specific AP-1-IRF complexes. Science 338, 975–980 (2012).
Li, P. et al. BATF-JUN is critical for IRF4-mediated transcription in T cells. Nature 490, 543–546 (2012).
Ciofani, M. et al. A validated regulatory network for Th17 cell specification. Cell 151, 289–303 (2012).
Wang, J. et al. A differentiation checkpoint limits hematopoietic stem cell self-renewal in response to DNA damage. Cell 148, 1001–1014 (2012).
Man, K. et al. The transcription factor IRF4 is essential for TCR affinity-mediated metabolic programming and clonal expansion of T cells. Nat. Immunol. 14, 1155–1165 (2013).
Ernst, J. et al. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature 473, 43–49 (2011).
Ashburner, M. et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 25, 25–29 (2000).
Lau, L.F. & Nathans, D. Expression of a set of growth-related immediate early genes in BALB/c 3T3 cells: coordinate regulation with c-fos or c-myc. Proc. Natl. Acad. Sci. USA 84, 1182–1186 (1987).
Paley, M.A. et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science 338, 1220–1225 (2012).
Kuroda, S. et al. Basic leucine zipper transcription factor, ATF-like (BATF) regulates epigenetically and energetically effector CD8 T-cell differentiation via Sirt1 expression. Proc. Natl. Acad. Sci. USA 108, 14885–14889 (2011).
Cruz-Guilloty, F. et al. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. J. Exp. Med. 206, 51–59 (2009).
Yosef, N. & Regev, A. Impulse control: temporal dynamics in gene transcription. Cell 144, 886–896 (2011).
Mangan, S., Itzkovitz, S., Zaslaver, A. & Alon, U. The incoherent feed-forward loop accelerates the response-time of the gal system of Escherichia coli. J. Mol. Biol. 356, 1073–1081 (2006).
Mangan, S. & Alon, U. Structure and function of the feed-forward loop network motif. Proc. Natl. Acad. Sci. USA 100, 11980–11985 (2003).
Yao, S. et al. Interferon regulatory factor 4 sustains CD8+ T cell expansion and effector differentiation. Immunity 39, 833–845 (2013).
Tussiwand, R. et al. Compensatory dendritic cell development mediated by BATF-IRF interactions. Nature 490, 502–507 (2012).
Kolumam, G.A., Thomas, S., Thompson, L.J., Sprent, J. & Murali-Krishna, K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 202, 637–650 (2005).
Vahedi, G. et al. STATs shape the active enhancer landscape of T cell populations. Cell 151, 981–993 (2012).
Kao, C. et al. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat. Immunol. 12, 663–671 (2011).
Kurachi, M. et al. Chemokine receptor CXCR3 facilitates CD8+ T cell differentiation into short-lived effector cells leading to memory degeneration. J. Exp. Med. 208, 1605–1620 (2011).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Kharchenko, P.V., Tolstorukov, M.Y. & Park, P.J. Design and analysis of ChIP-seq experiments for DNA-binding proteins. Nat. Biotechnol. 26, 1351–1359 (2008).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Quinlan, A.R. & Hall, I.M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010).
Landt, S.G. et al. ChIP-seq guidelines and practices of the ENCODE and modENCODE consortia. Genome Res. 22, 1813–1831 (2012).
Machanick, P. & Bailey, T.L. MEME-ChIP: motif analysis of large DNA datasets. Bioinformatics 27, 1696–1697 (2011).
Team, R.D.C. R: A language and environment for statistical computing. R Foundation for Statistical Computing: Vienna, Austria, 2009.
Irizarry, R.A. et al. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 31, e15 (2003).
Bolstad, B.M., Irizarry, R.A., Astrand, M. & Speed, T.P. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19, 185–193 (2003).
Irizarry, R.A. et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4, 249–264 (2003).
Linhart, C., Halperin, Y. & Shamir, R. Transcription factor and microRNA motif discovery: the Amadeus platform and a compendium of metazoan target sets. Genome Res. 18, 1180–1189 (2008).
Zheng, G. et al. ITFP: an integrated platform of mammalian transcription factors. Bioinformatics 24, 2416–2417 (2008).
Wilson, N.K. et al. Combinatorial transcriptional control in blood stem/progenitor cells: genome-wide analysis of ten major transcriptional regulators. Cell Stem Cell 7, 532–544 (2010).
Lachmann, A. et al. transcription factor regulation inferred from integrating genome-wide ChIP-X experiments. Bioinformatics 26, 2438–2444 (2010).
Liberzon, A. et al. Molecular signatures database (MSigDB) 3.0. Bioinformatics 27, 1739–1740 (2011).
Jiang, C., Xuan, Z., Zhao, F. & Zhang, M.Q. TRED: a transcriptional regulatory element database, new entries and other development. Nucleic Acids Res. 35 Database issue, D137–D140 (2007).
Awasthi, A. et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat. Immunol. 8, 1380–1389 (2007).
Xiao, S. et al. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-β-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J. Immunol. 181, 2277–2284 (2008).
Amit, I. et al. Unbiased reconstruction of a mammalian transcriptional network mediating pathogen responses. Science 326, 257–263 (2009).
Jux, B., Kadow, S. & Esser, C. Langerhans cell maturation and contact hypersensitivity are impaired in aryl hydrocarbon receptor-null mice. J. Immunol. 182, 6709–6717 (2009).
Durant, L. et al. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity 32, 605–615 (2010).
Yang, X.P. et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat. Immunol. 12, 247–254 (2011).
Shi, L.Z. et al. HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med. 208, 1367–1376 (2011).
Yosef, N. et al. Dynamic regulatory network controlling TH17 cell differentiation. Nature 496, 461–468 (2013).
Acknowledgements
We thank M. Ali for animal care; K. Mansfield and J. Kurachi for technical assistance; the Center for Cancer Computational Biology Sequencing Core of the Dana-Farber Cancer Institute for sequencing services and advice; and members of the Haining and Wherry laboratories for discussions. Supported by the US National Institutes of Health (T32 AI00762 to P.M.O.; AI091493 to W.N.H.; AI083022, AI095608 and HHSN266200500030C to E.J.W.; and AI082630 to W.N.H. and E.J.W.), the US Government (Presidential Early Career Award for Science and Engineering to W.N.H.), The Ministry of Education, Culture, Sports, Science and Technology of Japan (Grants-in-Aid for Young Scientists (B) 22790453 to M.K.), the Uehara Memorial Foundation of Japan (M.K.) and the German National Academic Foundation (M.G.K.).
Author information
Authors and Affiliations
Contributions
M.K. and P.M.O. did the experiments for the animal models; R.A.B., M.A.D., K.Y., J.G. and M.G.K. did the gene-expression and ChIP experiments; N.Y. and M.E.L. designed and did analytic experiments; A.R., E.J.W. and W.N.H. designed the analytic experiments; E.J.W. and W.N.H. conceived of the project; and M.K., R.A.B., E.J.W. and W.N.H. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
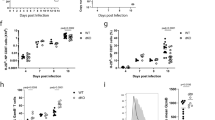
Supplementary Figure 1 Antigen specific CD8+ and CD4+ T cell responses are substantially diminished in Batf−/− mice.
Batf−/−, Batf+/−, and Batf+/+ mice (C57Bl/6 background) were intraperitoneally infected with LCMV Arm (2×105 pfu) and analyzed at indicated time points, as indicated in Fig. 1. (a-c) Histogram plots gated on gp33-specific Batf−/− (red) and Batf+/+ (black) CD8+ T cells showing expression of CD27, CXCR3, CD25, KLRG1 and CD127 (a), and granzyme B (b), and T-bet and Eomes (c) at d8 and 40 p.i. Graph shows MFI of T-bet and Eomes (Mean ± s.e.m) (c). (d-g) The antigen-specific CD4+ T cell response is also diminished in Batf−/− mice. (d) Flow cytometry plots gated on CD4+ cells showing percentage of gp66-specific cells at d8 and 40 p.i. in the blood. (e) Number of gp66-specific CD4+ T cells per 1×106 cells in the blood. Mean ± s.e.m. (f) Number of gp66-specific CD4+ T cells at d8 in the spleen. Mean ± s.e.m. (g) Histogram plots gated on gp66-specific Batf−/− (red) and Batf+/+ (black) CD4+ T cells showing expression of Ly6C, CD127, PSGL1 and CD29 at d8 and 40 p.i. Data are representative of 2 independent experiments (n=3-5 per time point). *P < 0.005, **P < 0.001, ***P < 0.0001 (unpaired Student's t-test).
Supplementary Figure 2 Similar naive phenotype of Batf−/− and Batf+/+ P14 cells at transfer and no evidence for rejection of Batf−/− P14 cells.
(a) Batf−/− and Batf+/+ P14 cells showed similar naive phenotype at adoptive transfer. Splenic Batf−/− and Batf+/+ P14 cells from 6-10 weeks age donor mice were analyzed for representative T cell surface markers. Batf−/− and Batf+/+ P14 cells showed similar naive phenotype as no ~ low expression of CD25, CD69, CXCR3, CD27, CD43, KLRG1 and high expression of CD62L and CD127. (b) Batf−/− P14 cells persist in the recipient in the absence of infection. Naive Batf−/− (CD45.2+) and Batf+/+ (CD45.1+CD45.2+) P14 cells (total 1×106 cells) were mixed at 1:1, labeled with Cell Trace Violet and adoptively transferred into non-irradiated CD45.1+ hosts. The recipient mice were analyzed at d1 and d42 after transfer. Both Batf−/− and Batf+/+ P14 cells persisted equally well in vivo and retained similar naive phenotype at d42. Representative flow plots gated on P14 cells in the spleen are shown (n=3-4 per time point). Representative of two independent experiments (n=3-4).
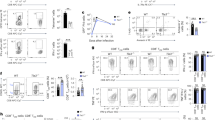
Supplementary Figure 3 No evidence for competition with Batf+/+ P14 cell or skewed tissue distribution.
(a-b) Single transfer of Batf−/−, Batf+/−, and Batf+/+ P14 cells (1×104 cells) showed defective Batf−/− but normal Batf+/− CD8 T cell response after LCMV Arm infection. (a) Representative flow plots gated on PBMC at d8 p.i. (b) Number of P14 cells per 1x106 cells in the blood. Mean ± s.e.m. (c) Systemic analysis in the experiments described in Fig.2 a-b (total 1×104 P14 cells transfer) revealed no skewed tissue distribution of Batf−/− versus Batf+/+ P14 cells at both effector (d8) and memory (d224) phase. Mean ± s.e.m. *P < 0.05, **P<0.005, ***P<0.001, ****P<0.0005, *****P<0.0001. (d) Phenotypes of remaining d8 effector and d44 memory Batf−/− P14 cells are similar to Batf+/+ P14 cells. In the experiments described in Fig. 2, surface expression of indicated markers were examined at d8 and d44 p.i. Data are representative of 2 independent experiments (n=3-4 per time point).
Supplementary Figure 4 Diminished Batf−/− P14 cell response with Listeria infection and dendritic cell immunization.
Batf−/− and Batf+/+ P14 cells (total 1×104 cells) were mixed at 1:1 and adoptively transferred into CD45.1+ mice as described in Fig. 2. Next day, the recipient mice were intravenously infected with LMgp33 (1×104 cfu) (a-c) or immunized by i.v. injection of DCgp33 (1×106 cells) with graded dose of wild-type LM (0, 1×102 ~ 1×105 cfu) (d-f). (a) Flow cytometry plots gated on P14 cells showing percentage of Batf+/+ (upper, CD45.1+CD45.2+) and Batf−/− (lower, CD45.2+). Numbers in the plots show percent Batf−/− and Batf+/+ among total P14 cells. (b) Number of Batf−/− and Batf+/+ P14 cells per 1×106 cells in the blood. Mean ± s.e.m. (c) Number of Batf−/− and Batf+/+ P14 cells in the indicated tissues at d63 p.i. Mean ± s.e.m. *P<0.05, **P<0.005 (unpaired Student's t-test). (d) Representative flow plots gated on P14 cells at transfer and d7 p.i. (blood). Numbers in the plots show percent of Batf−/− and Batf+/+ among total P14 cells. (e) Numbers of Batf−/− and Batf+/+ P14 cells per 1×106 cells in the blood. Mean ± s.e.m. (f) Percentage of Batf−/− P14 cells among total P14 cells in the blood. Mean ± s.e.m. Data are representative of 2 independent experiments (n=4-5 per time point).
Supplementary Figure 5 Efficiency of RV transduction and BATF overexpression.
Efficiency of RV transduction of P14 cells and overexpression of BATF were assessed in vitro 2 days after stimulation (one day after RV transduction). Numbers in the plots gated on P14 cells indicate percent GFP+ (RV-transduced) cells (a), and BATF positive cells (b). Representative data from 4 independent experiments (n=3-5 per group).
Supplementary Figure 6 In vitro effectors and in vivo effectors exhibit significantly similar transcriptional activity.
Gene set enrichment analysis of an in vitro effector CD8+ T cell signature in LCMV-specific CD8+ T cell profiles. The top 100 genes in day 3 in vitro effectors compared to naive CD8+ T cells were tested for enrichment in the rank ordered list of genes differentially expressed in day 8 in vivo effectors from LCMV Arm infection versus naive CD8+ T cells.
Supplementary Figure 7 BATF binds DNA with the Jun proteins and increases the binding of IRF4.
(a) Venn diagrams of the overlap of BATF-bound regions with cJun- (left), JunB- (middle), and JunD-bound regions (right). Significance was assessed with a binomial probability test. (b) Analysis of the background-subtracted ChIP count density (ChIP density) for all BATF and IRF4-bound regions in three classes of regions: BATF-only, IRF4-only, and combined BATF+IRF4 regions. (c) ChIP density of BATF (left) and IRF4 (right) comparing the regions of only the respective TF with the combined BATF+IRF4 regions. *P<2.2×10-16 (Mann-Whitney Test).
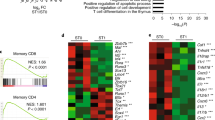
Supplementary Figure 8 Extended BATF transcription factor interaction network.
Incoming or outgoing connections are indicated by arrow direction; differential expression of TFs in Batf−/− effector CD8+ T cells is indicated by color of node and edge; and presence of shared target genes indicated by thick node outline. Nodes in grey are not differentially expressed in Batf−/− effector CD8+ T cells but are bound by or bind to BATF.
Supplementary Figure 9 Loss of BATF perturbs the earliest stages of effector differentiation.
(a) Histograms gated on P14 cells show expression of Bcl-2 and CD95. Experiment design is described as Fig. 7. Numbers in the plot show MFI (mean ± 2s.d.). Time points for analysis are shown above histogram. (b) BATF ChIP-PCR was performed in Batf+/+ (left) and Batf−/− (right) naive CD8+ T cells and CD8+ T cells after 1, 2, or 3 days of in vitro stimulation with anti-CD3 and anti-CD28 in the presence of recombinant human Il-2 for Bcl2 (●), Ctla4 (▴), Il2ra (▪), and Il10 (⋄). (c-f) Altered effector CD8+ T cell differentiation in the absence of Batf appears as early as d2 p.i. Experiment design is described as Fig. 7. (c) Extended panels for T cell activation and differentiation markers. (d) Histograms gated on P14 cells show expression of co-stimulatory molecules at d3. (e) CD62L and CD69 expression on each generation of effector P14 cells at d3. (f) TNF and MIP1α production upon gp33 peptide restimulation at d2.
Supplementary Figure 10 Model of a proposed BATF regulatory circuit.
BATF acts as an incoherent feed-forward circuit. TCR activation upregulates BATF which, together with IRF4 and Jun binding partners, feeds forward by positively regulating (green connections) cytokine receptors and effector transcription factors, but negatively regulates (red connections) effector molecules. BATF also provides negative feedback to IRF4 and Jun expression.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Supplementary Tables 1 and 2 (PDF 7412 kb)
Rights and permissions
About this article
Cite this article
Kurachi, M., Barnitz, R., Yosef, N. et al. The transcription factor BATF operates as an essential differentiation checkpoint in early effector CD8+ T cells. Nat Immunol 15, 373–383 (2014). https://doi.org/10.1038/ni.2834
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2834
This article is cited by
-
BATF and BATF3 deficiency alters CD8+ effector/exhausted T cells balance in skin transplantation
Molecular Medicine (2024)
-
FOXP1 and KLF2 reciprocally regulate checkpoints of stem-like to effector transition in CAR T cells
Nature Immunology (2024)
-
Reprogramming T cell differentiation and exhaustion in CAR-T cell therapy
Journal of Hematology & Oncology (2023)
-
Cardinal features of immune memory in innate lymphocytes
Nature Immunology (2023)
-
Glycogen synthase kinase 3 controls T-cell exhaustion by regulating NFAT activation
Cellular & Molecular Immunology (2023)