Abstract
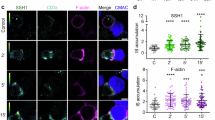
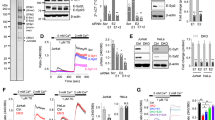
The mechanisms by which Lat (a key adaptor in the T cell antigen receptor (TCR) signaling pathway) and the TCR come together after TCR triggering are not well understood. We investigate here the role of SNARE proteins, which are part of protein complexes involved in the docking, priming and fusion of vesicles with opposing membranes, in this process. Here we found, by silencing approaches and genetically modified mice, that the vesicular SNARE VAMP7 was required for the recruitment of Lat-containing vesicles to TCR-activation sites. Our results indicated that this did not involve fusion of Lat-containing vesicles with the plasma membrane. VAMP7, which localized together with Lat on the subsynaptic vesicles, controlled the phosphorylation of Lat, formation of the TCR-Lat-signaling complex and, ultimately, activation of T cells. Our findings suggest that the transport and docking of Lat-containing vesicles with target membranes containing TCRs regulates TCR-induced signaling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Weiss, A. & Littman, D.R. Signal transduction by lymphocyte antigen receptors. Cell 76, 263–274 (1994).
Zhang, W., Trible, R.P. & Samelson, L.E. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity 9, 239–246 (1998).
Rudd, C.E. Adaptors and molecular scaffolds in immune cell signaling. Cell 96, 5–8 (1999).
Bunnell, S.C., Kapoor, V., Trible, R.P., Zhang, W. & Samelson, L.E. Dynamic actin polymerization drives T cell receptor-induced spreading: a role for the signal transduction adaptor LAT. Immunity 14, 315–329 (2001).
Bonello, G. et al. Dynamic recruitment of the adaptor protein LAT: LAT exists in two distinct intracellular pools and controls its own recruitment. J. Cell Sci. 117, 1009–1016 (2004).
Zhang, W. et al. Essential role of LAT in T cell development. Immunity 10, 323–332 (1999).
Zhang, W., Sloan-Lancaster, J., Kitchen, J., Trible, R.P. & Samelson, L.E. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell 92, 83–92 (1998).
Mingueneau, M. et al. Loss of the LAT adaptor converts antigen-responsive T cells into pathogenic effectors that function independently of the T cell receptor. Immunity 31, 197–208 (2009).
Roncagalli, R. et al. Lymphoproliferative disorders involving T helper effector cells with defective LAT signalosomes. Semin. Immunopathol. 32, 117–125 (2010).
Lillemeier, B.F. et al. TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nat. Immunol. 11, 90–96 (2010).
Purbhoo, M.A. et al. Dynamics of subsynaptic vesicles and surface microclusters at the immunological synapse. Sci. Signal. 3, ra36 (2010).
Williamson, D.J. et al. Pre-existing clusters of the adaptor Lat do not participate in early T cell signaling events. Nat. Immunol. 12, 655–662 (2011).
Südhof, T.C. The synaptic vesicle cycle revisited. Neuron 28, 317–320 (2000).
Risselada, H.J. & Grubmuller, H. How SNARE molecules mediate membrane fusion: recent insights from molecular simulations. Curr. Opin. Struct. Biol. 22, 187–196 (2012).
Proux-Gillardeaux, V., Rudge, R. & Galli, T. The tetanus neurotoxin-sensitive and insensitive routes to and from the plasma membrane: fast and slow pathways? Traffic 6, 366–373 (2005).
Danglot, L. et al. Absence of TI-VAMP/Vamp7 leads to increased anxiety in mice. J. Neurosci. 32, 1962–1968 (2012).
McMahon, H.T. et al. Cellubrevin is a ubiquitous tetanus-toxin substrate homologous to a putative synaptic vesicle fusion protein. Nature 364, 346–349 (1993).
Chaineau, M., Danglot, L. & Galli, T. Multiple roles of the vesicular-SNARE TI-VAMP in post-Golgi and endosomal trafficking. FEBS Lett. 583, 3817–3826 (2009).
Zhang, W. et al. Association of Grb2, Gads, and phospholipase C-γ1 with phosphorylated LAT tyrosine residues. Effect of LAT tyrosine mutations on T cell angigen receptor-mediated signaling. J. Biol. Chem. 275, 23355–23361 (2000).
Asada, H. et al. Grf40, A novel Grb2 family member, is involved in T cell signaling through interaction with SLP-76 and LAT. J. Exp. Med. 189, 1383–1390 (1999).
Harder, T. & Kuhn, M. Selective accumulation of raft-associated membrane protein LAT in T cell receptor signaling assemblies. J. Cell Biol. 151, 199–208 (2000).
Rao, S.K., Huynh, C., Proux-Gillardeaux, V., Galli, T. & Andrews, N.W. Identification of SNAREs involved in synaptotagmin VII-regulated lysosomal exocytosis. J. Biol. Chem. 279, 20471–20479 (2004).
Holt, O.J., Gallo, F. & Griffiths, G.M. Regulating secretory lysosomes. J. Biochem. 140, 7–12 (2006).
Das, V. et al. Activation-induced polarized recycling targets T cell antigen receptors to the immunological synapse; involvement of SNARE complexes. Immunity 20, 577–588 (2004).
Danglot, L. et al. Role of TI-VAMP and CD82 in EGFR cell-surface dynamics and signaling. J. Cell Sci. 123, 723–735 (2010).
Braun, V. et al. TI-VAMP/VAMP7 is required for optimal phagocytosis of opsonised particles in macrophages. EMBO J. 23, 4166–4176 (2004).
Mollinedo, F. et al. Combinatorial SNARE complexes modulate the secretion of cytoplasmic granules in human neutrophils. J. Immunol. 177, 2831–2841 (2006).
Sander, L.E. et al. Vesicle associated membrane protein (VAMP)-7 and VAMP-8, but not VAMP-2 or VAMP-3, are required for activation-induced degranulation of mature human mast cells. Eur. J. Immunol. 38, 855–863 (2008).
Marcet-Palacios, M. et al. Vesicle-associated membrane protein 7 (VAMP-7) is essential for target cell killing in a natural killer cell line. Biochem. Biophys. Res. Commun. 366, 617–623 (2008).
Douglass, A.D. & Vale, R.D. Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell 121, 937–950 (2005).
Sherman, E. et al. Functional nanoscale organization of signaling molecules downstream of the T cell antigen receptor. Immunity 35, 705–720 (2011).
Balagopalan, L. et al. c-Cbl-mediated regulation of LAT-nucleated signaling complexes. Mol. Cell Biol. 27, 8622–8636 (2007).
Vardhana, S., Choudhuri, K., Varma, R. & Dustin, M.L. Essential role of ubiquitin and TSG101 protein in formation and function of the central supramolecular activation cluster. Immunity 32, 531–540 (2010).
Alonso, R. et al. Diacylglycerol kinase α regulates the formation and polarisation of mature multivesicular bodies involved in the secretion of Fas ligand-containing exosomes in T lymphocytes. Cell Death Differ. 18, 1161–1173 (2011).
Kupfer, A., Dennert, G. & Singer, S.J. Polarization of the Golgi apparatus and the microtubule-organizing center within cloned natural killer cells bound to their targets. Proc. Natl. Acad. Sci. USA 80, 7224–7228 (1983).
Alcover, A. & Alarcon, B. Internalization and intracellular fate of TCR-CD3 complexes. Crit. Rev. Immunol. [In Process Citation] 20, 325–346 (2000).
Chaturvedi, A., Martz, R., Dorward, D., Waisberg, M. & Pierce, S.K. Endocytosed BCRs sequentially regulate MAPK and Akt signaling pathways from intracellular compartments. Nat. Immunol. 12, 1119–1126 (2011).
Burgo, A. et al. A Molecular network for the transport of the TI-VAMP/VAMP7 vesicles from cell center to periphery. Dev. Cell 23, 166–180 (2012).
Martín-Cófreces, N.B. et al. End-binding protein 1 controls signal propagation from the T cell receptor. EMBO J. 31, 4140–4152 (2012).
Antón, O.M., Andres-Delgado, L., Reglero-Real, N., Batista, A. & Alonso, M.A. MAL protein controls protein sorting at the supramolecular activation cluster of human T lymphocytes. J. Immunol. 186, 6345–6356 (2011).
Varma, R., Campi, G., Yokosuka, T., Saito, T. & Dustin, M.L. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity 25, 117–127 (2006).
Babich, A. et al. F-actin polymerization and retrograde flow drive sustained PLCgamma1 signaling during T cell activation. J. Cell Biol. 197, 775–787 (2012).
Chemin, K. et al. Cytokine secretion by CD4+ T cells at the immunological synapse requires Cdc42-dependent local actin remodeling but not microtubule organizing center polarity. J. Immunol. 189, 2159–2168 (2012).
Rak, G.D., Mace, E.M., Banerjee, P.P., Svitkina, T. & Orange, J.S. Natural killer cell lytic granule secretion occurs through a pervasive actin network at the immune synapse. PLoS Biol. 9, e1001151 (2011).
Brown, A.C. et al. Remodelling of cortical actin where lytic granules dock at natural killer cell immune synapses revealed by super-resolution microscopy. PLoS Biol. 9, e1001152 (2011).
Husson, J., Chemin, K., Bohineust, A., Hivroz, C. & Henry, N. Force generation upon T cell receptor engagement. PLoS ONE 6, e19680 (2011).
Blanchard, N., Di Bartolo, V. & Hivroz, C. In the immune synapse, ZAP-70 controls T cell polarization and recruitment of signaling proteins but not formation of the synaptic pattern. Immunity 17, 389–399 (2002).
Martinez-Arca, S., Alberts, P., Zahraoui, A., Louvard, D. & Galli, T. Role of tetanus neurotoxin insensitive vesicle-associated membrane protein (TI-VAMP) in vesicular transport mediating neurite outgrowth. J. Cell Biol. 149, 889–900 (2000).
Owen, D.M. et al. PALM imaging and cluster analysis of protein heterogeneity at the cell surface. J. Biophotonics 3, 446–454 (2010).
Benvenuti, F. et al. Dendritic cell maturation controls adhesion, synapse formation, and the duration of the interactions with naive T lymphocytes. J. Immunol. 172, 292–301 (2004).
Acknowledgements
We thank A.-M. Lennon and S. Amigorena for discussions; and P. Pierobon, V. Fraisier, P. Paul-Gilloteaux, L. Sengmanivong and the Nikon Imaging Centre at Institut Curie (Centre National de la Recherche Scientifique) for technical assistance with microscopy and image analysis. Supported by Fondation pour la Recherche Médicale (P.L. and K.C., and T.G.'s group), la Ligue contre le Cancer (J.-M.C. and A.B.), DC-Biol Labex (C.H.'s group), Institut National de la Santé et de la Recherche Médicale (T.G.'s group) and the Mairie de Paris Medical Research and Health Program (T.G.'s group).
Author information
Authors and Affiliations
Contributions
P.L. designed, did and analyzed three-dimensional microscopy, TIRF microscopy and biochemistry experiments, and prepared the manuscript; D.J.W. designed, did and analyzed PALM experiments and revised the manuscript; J.-M.C. did and analyzed experiments with mouse T cells; S.D. designed vectors for and did mice genotyping; K.C. and A.B. designed and analyzed three-dimensional microscopy experiments; L.D. and T.G. provided VAMP7-deficient mice and tools; K.G. designed PALM experiments and assisted with manuscript preparation; T.G. designed VAMP7-deficient mice, discussed the results and revised the manuscript; and C.H. conceived of the study, did biochemistry experiments and prepared manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 (PDF 3445 kb)
Supplementary Video 1
Lat subsynaptic vesicles are recruited to the immunological synapse in activated control Jurkat cells. Time lapse TIRF microscopy images of Lat-GFP expressing Jurkat cells infected with a control shRNA encoding lentiviruses put on glass slides coated with anti-CD3 and anti-CD28 mAbs. (AVI 3503 kb)
Supplementary Video 2
VAMP7 silencing inhibits the recruitment of Lat subsynaptic vesicles to the immunological synapse. Time lapse TIRF microscopy images of Lat-GFP expressing Jurkat cells infected with the VAMP7 specific Sh1RNA encoding lentiviruses put on glass slides coated with anti-CD3 and anti-CD28 mAbs. (AVI 4789 kb)
Supplementary Video 3
VAMP7 silencing inhibits the recruitment of Lat subsynaptic vesicles to the immunological synapse. Time lapse TIRF microscopy images of Lat-GFP expressing Jurkat cells infected with the VAMP7 specific Sh5RNA encoding lentiviruses put on glass slides coated with anti-CD3 and anti-CD28 mAbs. (AVI 2722 kb)
Supplementary Video 4
Lat subsynaptic vesicles recruited to the immunological synapse contain VAMP7.Time-lapse TIRF microscopy images of Jurkat cells co-transfected with Lat-mCherry (magenta) and GFP-VAMP7 (green) put on glass slides coated with anti-CD3 and anti-anti-CD28 mAbs. Left: GFP-VAMP7, middle: Lat-mCherry, right: merge. (AVI 6222 kb)
Rights and permissions
About this article
Cite this article
Larghi, P., Williamson, D., Carpier, JM. et al. VAMP7 controls T cell activation by regulating the recruitment and phosphorylation of vesicular Lat at TCR-activation sites. Nat Immunol 14, 723–731 (2013). https://doi.org/10.1038/ni.2609
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2609