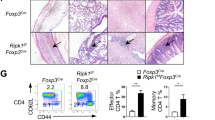
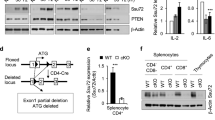
Abstract
The maintenance of immune homeostasis requires regulatory T cells (Treg cells). Here we found that Treg cell–specific ablation of Ubc13, a Lys63 (K63)-specific ubiquitin-conjugating enzyme, caused aberrant T cell activation and autoimmunity. Although Ubc13 deficiency did not affect the survival of Treg cells or expression of the transcription factor Foxp3, it impaired the in vivo suppressive function of Treg cells and rendered them sensitive to the acquisition of T helper type 1 (TH1) cell– and interleukin 17 (IL-17)-producing helper T (TH17) cell–like effector phenotypes. This function of Ubc13 involved its downstream target, the kinase IKK. The Ubc13-IKK signaling axis controlled the expression of specific Treg cell effector molecules, including IL-10 and SOCS1. Collectively, our findings suggest that the Ubc13-IKK signaling axis regulates the molecular program that maintains Treg cell function and prevents Treg cells from acquiring inflammatory phenotypes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Sakaguchi, S., Yamaguchi, T., Nomura, T. & Ono, M. Regulatory T cells and immune tolerance. Cell 133, 775–787 (2008).
Rudensky, A.Y. Regulatory T cells and Foxp3. Immunol. Rev. 241, 260–268 (2011).
Shevach, E.M. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity 30, 636–645 (2009).
Rubtsov, Y.P. et al. Stability of the regulatory T cell lineage in vivo. Science 329, 1667–1671 (2010).
Bailey-Bucktrout, S.L. & Bluestone, J.A. Regulatory T cells: stability revisited. Trends Immunol. 32, 301–306 (2011).
Hori, S. Regulatory T cell plasticity: beyond the controversies. Trends Immunol. 32, 295–300 (2011).
Yang, X.O. et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity 29, 44–56 (2008).
Duarte, J.H., Zelenay, S., Bergman, M.L., Martins, A.C. & Demengeot, J. Natural Treg cells spontaneously differentiate into pathogenic helper cells in lymphopenic conditions. Eur. J. Immunol. 39, 948–955 (2009).
Tsuji, M. et al. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer's patches. Science 323, 1488–1492 (2009).
Zhou, X. et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 10, 1000–1007 (2009).
Ayyoub, M. et al. Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the TH17 lineage-specific transcription factor RORγt. Proc. Natl. Acad. Sci. USA 106, 8635–8640 (2009).
Voo, K.S. et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc. Natl. Acad. Sci. USA 106, 4793–4798 (2009).
Takahashi, R. et al. SOCS1 is essential for regulatory T cell functions by preventing loss of Foxp3 expression as well as IFN-γ and IL-17A production. J. Exp. Med. 208, 2055–2067 (2011).
Xu, L., Kitani, A., Fuss, I. & Strober, W. Cutting edge: regulatory T cells induce CD4+CD25−Foxp3− T cells or are self-induced to become TH17 cells in the absence of exogenous TGF-β. J. Immunol. 178, 6725–6729 (2007).
Collins, E.L. et al. Inhibition of SOCS1−/− lethal autoinflammatory disease correlated to enhanced peripheral Foxp3+ regulatory T cell homeostasis. J. Immunol. 187, 2666–2676 (2011).
Sakaguchi, S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22, 531–562 (2004).
Bhoj, V.G. & Chen, Z.J. Ubiquitylation in innate and adaptive immunity. Nature 458, 430–437 (2009).
Sun, L., Deng, L., Ea, C.-K., Xia, Z.-P. & Chen, Z.J. The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol. Cell 14, 289–301 (2004).
Yamamoto, M. et al. Cutting edge: pivotal function of Ubc13 in thymocyte TCR signaling. J. Immunol. 177, 7520–7524 (2006).
Isomura, I. et al. c-Rel is required for the development of thymic Foxp3+ CD4 regulatory T cells. J. Exp. Med. 206, 3001–3014 (2009).
Long, M., Park, S.G., Strickland, I., Hayden, M.S. & Ghosh, S. Nuclear factor-κB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor. Immunity 31, 921–931 (2009).
Ruan, Q. et al. Development of Foxp3+ regulatory t cells is driven by the c-Rel enhanceosome. Immunity 31, 932–940 (2009).
Zheng, Y. et al. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature 463, 808–812 (2010).
Yamamoto, M. et al. Key function for the Ubc13 E2 ubiquitin-conjugating enzyme in immune receptor signaling. Nat. Immunol. 7, 962–970 (2006).
Zhou, X. et al. Selective miRNA disruption in Treg cells leads to uncontrolled autoimmunity. J. Exp. Med. 205, 1983–1991 (2008).
Srinivas, S. et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4 (2001).
Morrissey, P.J., Charrier, K., Braddy, S., Liggitt, D. & Watson, J.D. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+ T cells. J. Exp. Med. 178, 237–244 (1993).
Mattioli, I. et al. Transient and selective NF-κB p65 serine 536 phosphorylation induced by T cell costimulation is mediated by IκB kinase β and controls the kinetics of p65 nuclear import. J. Immunol. 172, 6336–6344 (2004).
Sasaki, Y. et al. Canonical NF-κB activity, dispensable for B cell development, replaces BAFF-receptor signals and promotes B cell proliferation upon activation. Immunity 24, 729–739 (2006).
Pasparakis, M. et al. TNF-mediated inflammatory skin disease in mice with epidermis-specific deletion of IKK2. Nature 417, 861–866 (2002).
Rubtsov, Y.P. et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 28, 546–558 (2008).
Chaudhry, A. et al. Interleukin-10 signaling in regulatory T cells is required for suppression of TH17 cell-mediated inflammation. Immunity 34, 566–578 (2011).
Huber, S. et al. TH17 cells express interleukin-10 receptor and are controlled by Foxp3− and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity 34, 554–565 (2011).
Pillemer, B.B., Xu, H., Oriss, T.B., Qi, Z. & Ray, A. Deficient SOCS3 expression in CD4+CD25+FoxP3+ regulatory T cells and SOCS3-mediated suppression of Treg function. Eur. J. Immunol. 37, 2082–2089 (2007).
Cao, S., Zhang, X., Edwards, J.P. & Mosser, D.M. NF-κB1 (p50) homodimers differentially regulate pro- and anti-inflammatory cytokines in macrophages. J. Biol. Chem. 281, 26041–26050 (2006).
Benkhart, E.M., Siedlar, M., Wedel, A., Werner, T. & Ziegler-Heitbrock, H.W. Role of Stat3 in lipopolysaccharide-induced IL-10 gene expression. J. Immunol. 165, 1612–1617 (2000).
Saito, H. et al. IFN regulatory factor-1-mediated transcriptional activation of mouse STAT-induced STAT inhibitor-1 gene promoter by IFN-γ. J. Immunol. 164, 5833–5843 (2000).
Diehl, S. et al. Inhibition of TH1 differentiation by IL-6 is mediated by SOCS1. Immunity 13, 805–815 (2000).
Charoenthongtrakul, S., Zhou, Q., Shembade, N., Harhaj, N.S. & Harhaj, E.W. Human T cell leukemia virus type 1 Tax inhibits innate antiviral signaling via NF-κB-dependent induction of SOCS1. J. Virol. 85, 6955–6962 (2011).
Flowers, L.O. et al. Characterization of a peptide inhibitor of Janus kinase 2 that mimics suppressor of cytokine signaling 1 function. J. Immunol. 172, 7510–7518 (2004).
Flowers, L.O., Subramaniam, P.S. & Johnson, H.M.A. SOCS-1 peptide mimetic inhibits both constitutive and IL-6 induced activation of STAT3 in prostate cancer cells. Oncogene 24, 2114–2120 (2005).
Jager, L.D. et al. The kinase inhibitory region of SOCS-1 is sufficient to inhibit T-helper 17 and other immune functions in experimental allergic encephalomyelitis. J. Neuroimmunol. 232, 108–118 (2011).
Kryczek, I. et al. IL-17+ regulatory T cells in the microenvironments of chronic inflammation and cancer. J. Immunol. 186, 4388–4395 (2011).
Chang, M. et al. The ubiquitin ligase Peli1 negatively regulates T cell activation and prevents autoimmunity. Nat. Immunol. 12, 1002–1009 (2011).
Harhaj, E.W., Maggirwar, S.B. & Sun, S.-C. Inhibition of p105 processing by NF-κB proteins in transiently transfected cells. Oncogene 12, 2385–2392 (1996).
Acknowledgements
We thank M. Karin (University of California, San Diego) for the IKK2(CA) expression vector; T. Kishimoto (Osaka University) for the mouse Socs1-luc reporter; and personnel from the flow cytometry, DNA analysis, and histology core facilities at MD Anderson Cancer Center for technical assistance. This work is supported by the US National Institutes of Health (AI057555, AI064639 and GM84459) and the G.S. Hogan Gastrointestinal Cancer Research Fund.
Author information
Authors and Affiliations
Contributions
J.-H.C. designed and did the research, prepared the figures, and wrote part of the manuscript; Y.X. did the luciferase assays; X.Z. provided technical help for adoptive transfer; H.H. did the Ubc13 immunoblot analysis; J.J. constructed the mutant Socs1 luciferase plasmid; J.Y., X.C. and X.W. contributed to the generation of mouse models; H.M.J., S.A. and M.P. contributed reagents; and S.-C.S. designed the research and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–12 and Table 1 (PDF 19955 kb)
Rights and permissions
About this article
Cite this article
Chang, JH., Xiao, Y., Hu, H. et al. Ubc13 maintains the suppressive function of regulatory T cells and prevents their conversion into effector-like T cells. Nat Immunol 13, 481–490 (2012). https://doi.org/10.1038/ni.2267
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2267