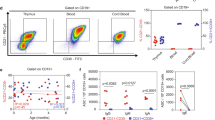
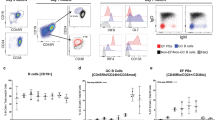
Abstract
Neutrophils use immunoglobulins to clear antigen, but their role in immunoglobulin production is unknown. Here we identified neutrophils around the marginal zone (MZ) of the spleen, a B cell area specialized in T cell–independent immunoglobulin responses to circulating antigen. Neutrophils colonized peri-MZ areas after postnatal mucosal colonization by microbes and enhanced their B cell–helper function after receiving reprogramming signals, including interleukin 10 (IL-10), from splenic sinusoidal endothelial cells. Splenic neutrophils induced immunoglobulin class switching, somatic hypermutation and antibody production by activating MZ B cells through a mechanism that involved the cytokines BAFF, APRIL and IL-21. Neutropenic patients had fewer and hypomutated MZ B cells and a lower abundance of preimmune immunoglobulins to T cell–independent antigens, which indicates that neutrophils generate an innate layer of antimicrobial immunoglobulin defense by interacting with MZ B cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
12 July 2013
In the version of this article initially published, the second affiliation for Stephanie C. Ganal is missing. This author is also affiliated with the Spemann Graduate School of Biology and Medicine, Freiburg, Germany. The error has been corrected in the HTML and PDF versions of the article.
References
Nathan, C. Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 6, 173–182 (2006).
Soehnlein, O. An elegant defense: how neutrophils shape the immune response. Trends Immunol. 30, 511–512 (2009).
Brinkmann, V. et al. Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 (2004).
Mantovani, A., Cassatella, M.A., Costantini, C. & Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 11, 519–531 (2011).
Yang, D., de la Rosa, G., Tewary, P. & Oppenheim, J.J. Alarmins link neutrophils and dendritic cells. Trends Immunol. 30, 531–537 (2009).
van Gisbergen, K.P., Geijtenbeek, T.B. & van Kooyk, Y. Close encounters of neutrophils and DCs. Trends Immunol. 26, 626–631 (2005).
Gabrilovich, D.I. & Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174 (2009).
Zhang, X., Majlessi, L., Deriaud, E., Leclerc, C. & Lo-Man, R. Coactivation of Syk kinase and MyD88 adaptor protein pathways by bacteria promotes regulatory properties of neutrophils. Immunity 31, 761–771 (2009).
Pasquier, B. et al. Identification of FcαRI as an inhibitory receptor that controls inflammation: dual role of FcRgγ ITAM. Immunity 22, 31–42 (2005).
Tsuboi, N., Asano, K., Lauterbach, M. & Mayadas, T.N. Human neutrophil Fcγ receptors initiate and play specialized nonredundant roles in antibody-mediated inflammatory diseases. Immunity 28, 833–846 (2008).
Scapini, P. et al. G-CSF-stimulated neutrophils are a prominent source of functional BLyS. J. Exp. Med. 197, 297–302 (2003).
Huard, B. et al. APRIL secreted by neutrophils binds to heparan sulfate proteoglycans to create plasma cell niches in human mucosa. J. Clin. Invest. 118, 2887–2895 (2008).
Mackay, F. & Schneider, P. Cracking the BAFF code. Nat. Rev. Immunol. 9, 491–502 (2009).
Schneider, P. et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J. Exp. Med. 189, 1747–1756 (1999).
Litinskiy, M.B. et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 3, 822–829 (2002).
He, B. et al. Intestinal bacteria trigger T cell-independent immunoglobulin A2 class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity 26, 812–826 (2007).
Xu, W. et al. Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI. Nat. Immunol. 8, 294–303 (2007).
He, B. et al. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nat. Immunol. 11, 836–845 (2010).
Cerutti, A., Chen, K. & Chorny, A. Immunoglobulin responses at the mucosal interface. Annu. Rev. Immunol. 29, 273–293 (2011).
Martin, F. & Kearney, J.F. Marginal-zone B cells. Nat. Rev. Immunol. 2, 323–335 (2002).
Weill, J.C., Weller, S. & Reynaud, C.A. Human marginal zone B cells. Annu. Rev. Immunol. 27, 267–285 (2009).
Berkowska, M.A. et al. Human memory B cells originate from three distinct germinal center-dependent and -independent maturation pathways. Blood 118, 2150–2158 (2011).
Krueger, J.M. et al. Peptidoglycans as promoters of slow-wave sleep. II. Somnogenic and pyrogenic activities of some naturally occurring muramyl peptides; correlations with mass spectrometric structure determination. J. Biol. Chem. 259, 12659–12662 (1984).
Brenchley, J.M. et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 12, 1365–1371 (2006).
Clarke, T.B. et al. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat. Med. 16, 228–231 (2010).
Haas, A. et al. Systemic antibody responses to gut commensal bacteria during chronic HIV-1 infection. Gut 60, 1506–1519 (2011).
Weller, S. et al. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood 104, 3647–3654 (2004).
Scheeren, F.A. et al. T cell-independent development and induction of somatic hypermutation in human IgM+IgD+CD27+ B cells. J. Exp. Med. 205, 2033–2042 (2008).
Balázs, M., Martin, F., Zhou, T. & Kearney, J. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity 17, 341–352 (2002).
Honjo, T., Kinoshita, K. & Muramatsu, M. Molecular mechanism of class switch recombination: linkage with somatic hypermutation. Annu. Rev. Immunol. 20, 165–196 (2002).
Muramatsu, M. et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 102, 553–563 (2000).
Han, C. et al. Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nat. Immunol. 11, 734–742 (2010).
Liu, Y., Chen, G.Y. & Zheng, P. CD24-Siglec G/10 discriminates danger- from pathogen-associated molecular patterns. Trends Immunol. 30, 557–561 (2009).
DiStasi, M.R. & Ley, K. Opening the flood-gates: how neutrophil-endothelial interactions regulate permeability. Trends Immunol. 30, 547–556 (2009).
Avery, D.T. et al. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. J. Exp. Med. 207, 155–171 (2010).
Cerutti, A., Puga, I. & Cols, M. Innate control of B cell responses. Trends Immunol. 32, 202–211 (2011).
Zhu, J. et al. Conversion of proepithelin to epithelins: roles of SLPI and elastase in host defense and wound repair. Cell 111, 867–878 (2002).
Mansell, A. et al. Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nat. Immunol. 7, 148–155 (2006).
Tang, W. et al. The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science 332, 478–484 (2011).
Klein, C. Genetic defects in severe congenital neutropenia: emerging insights into life and death of human neutrophil granulocytes. Annu. Rev. Immunol. 29, 399–413 (2011).
Donadieu, J., Fenneteau, O., Beaupain, B., Mahlaoui, N. & Chantelot, C.B. Congenital neutropenia: diagnosis, molecular bases and patient management. Orphanet J. Rare Dis. 6, 26 (2011).
Chen, K. et al. Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat. Immunol. 10, 889–898 (2009).
Chu, V.T. et al. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat. Immunol. 12, 151–159 (2011).
Macpherson, A.J. & Uhr, T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 303, 1662–1665 (2004).
De Santo, C. et al. Invariant NKT cells modulate the suppressive activity of IL-10-secreting neutrophils differentiated with serum amyloid A. Nat. Immunol. 11, 1039–1046 (2010).
Bernasconi, N.L., Traggiai, E. & Lanzavecchia, A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science 298, 2199–2202 (2002).
Cinamon, G. et al. Sphingosine 1-phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nat. Immunol. 5, 713–720 (2004).
Zhang, Q. et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464, 104–107 (2010).
Obata, T. et al. Indigenous opportunistic bacteria inhabit mammalian gut-associated lymphoid tissues and share a mucosal antibody-mediated symbiosis. Proc. Natl. Acad. Sci. USA 107, 7419–7424 (2010).
Cerutti, A. et al. CD40 ligand and appropriate cytokines induce switching to IgG, IgA, and IgE and coordinated germinal center-like phenotype differentiation in a human monoclonal IgM+IgD+ B cell line. J. Immunol. 160, 2145–2157 (1998).
Acknowledgements
We thank J. Farrés, J. Yélamos, A. Muntasell and M. López-Botet (Institut Municipal d'Investigació Mèdica-Hospital del Mar) for reagents, samples and discussions; N. Romo, S. Bascones, E. Ramirez and O. Fornas for help with cell sorting; and S. Mojal for help with the statistical analysis. Supported by Ministerio de Ciencia e Innovación (SAF 2008-02725 to A.Ce.), the US National Institutes of Health (R01 AI074378, P01 AI61093, U01 AI95613 and P01 096187 to A.Ce.), the European Commission (EUROPADnet HEALTH-F2-2008-201549 to A.Ce.), the Juan de la Cierva Program (I.P. and G.M.), the Instituto de Salud Carlos III (C.M.B. and M.G. and A.Ch.), the Ministerio de Ciencia e Innovación (C.M.B. and M.G.), Yerkes National Primate Research Center (P51 RR00165 to G.S.) and Fondazione C. Golgi di Brescia, Associazione Immunodeficienze Primitive (A.P.).
Author information
Authors and Affiliations
Contributions
I.P. and M.Co. designed and did research, discussed data and wrote the paper; C.M.B., B.H. and K.C. designed and did research; L.Ca., M.G., L.Co., A.Cho., M.S., W.X., G.M., A.Chi, T.B. and S.C.G. did research and discussed data; D.M.K., W.T., J.B.B., S.S., J.A.L., B.B., J.L., N.J., F.A., C.D.d.H., N.T., A.Ca., M.T., C.F., V.C., C.C., G.A.D., J.M.B., C.-M.F., G.S., C.C.-R., M.Ca., C.Du., L.D.N., V.L., A.P., J.-L.C., A.D., J.I.A., M.J., J.Y., N.M. and J.D. provided blood and tissue samples and discussed data; and A.Ce. designed research, discussed data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–22 and Tables 1–6 (PDF 2223 kb)
Supplementary Video 1
NBH cells form NET-like structures to interact with splenic MZ B cells. Confocal microscopy and three-dimensional animation of a spleen section stained for elastase (green), IgD (red) and DNA (blue). The movie was generated by acquiring up to 14 x,y planes with 0.3 μm z spacing. Original magnification, x40. One of three experiments yielding similar results. (AVI 17483 kb)
Supplementary Video 2
NBH cells spontaneously form DNA-containing NET-like projections. Confocal microscopy and three-dimensional animation of NBH cells stained for elastase (green) and DNA (blue). The movie was generated by acquiring up to 25 x,y planes with 0.3 μm z spacing. Original magnification, x63; digital magnification, x2. One of three experiments yielding similar results. (AVI 9943 kb)
Rights and permissions
About this article
Cite this article
Puga, I., Cols, M., Barra, C. et al. B cell–helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol 13, 170–180 (2012). https://doi.org/10.1038/ni.2194
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2194