Abstract
The transcriptional repressor Blimp-1 promotes the differentiation of CD8+ T cells into short-lived effector cells (SLECs) that express the lectin-like receptor KLRG-1, but how it operates remains poorly defined. Here we show that Blimp-1 bound to and repressed the promoter of the gene encoding the DNA-binding inhibitor Id3 in SLECs. Repression of Id3 by Blimp-1 was dispensable for SLEC development but limited the ability of SLECs to persist as memory cells. Enforced expression of Id3 was sufficient to restore SLEC survival and enhanced recall responses. Id3 function was mediated in part through inhibition of the transcriptional activity of E2A and induction of genes regulating genome stability. Our findings identify the Blimp-1–Id3–E2A axis as a key molecular switch that determines whether effector CD8+ T cells are programmed to die or enter the memory pool.
Similar content being viewed by others
Main
Blimp-1 is a transcriptional repressor originally described as programming the differentiation of B cells into end-stage, immunoglobulin-secreting plasma cells1. Blimp-1 has now emerged as a key master regulator of terminal differentiation in a variety of cell types, including keratinocytes and osteoclasts, as well as T lymphocytes2,3,4. In CD8+ T cells, Blimp-1 is required for differentiation into cytolytic effector T cells that express perforin and granzyme B and is necessary for efficient clearance of viral infection5,6,7. Short-lived effector cells (SLECs) that express the lectin-like receptor KLRG-1 have more Blimp-1 than do KLRG-1− memory precursor effector cells (MPECs) that express the interleukin 7 receptor α-chain (IL-7Rα)6,7,8,9. In the absence of Blimp-1, CD8+ T cells develop mainly into MPECs, which results in more formation of long-lived central memory T cells that express the activation marker CD62L (L-selectin) and release interleukin 2 (IL-2)5,6,7.
Although it is clear that Blimp-1 can inhibit the formation of memory CD8+ T cells by driving T cells to senescence5,6,7,10,11, the molecular mechanisms downstream of Blimp-1 remain poorly defined. At present only a few genes have been characterized as direct targets of Blimp-1 in T cells, including Bcl6, Il2 and Fos, which encode molecules linked to the homeostasis and long-term persistence of CD8+ T cells12,13,14. Here we show that, as in B cells15, Blimp-1 directly bound the promoter of the gene encoding the DNA-binding inhibitor Id3 (an antagonist of E-protein transcription factors) and repressed its expression in effector CD8+ T lymphocytes. Repression of Id3 by Blimp-1 enhanced the transcriptional activity of E2A, a member of the E-protein family, and was a critical determinant of whether effector CD8+ T cells were destined to die or enter into the memory pool.
Results
Blimp-1 represses Id3 expression in effector T cells
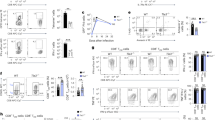
Transcriptome analysis of effector CD8+ T cell subsets has shown that Id3 is the transcription factor most upregulated in MPECs relative to its expression in SLECs6. Furthermore, effector CD8+ T cells that lack Blimp-1 also have higher expression of Id3 than do wild-type-cells6. We sought to further investigate the relationship between Blimp-1 and Id3 in CD8+ T cells by evaluating their expression during immune responses to a viral infection. We adoptively transferred pmel-1 CD8+ T cells (which recognize the shared melanoma-melanocyte differentiation antigen gp100)16 into wild-type mice and evaluated expression of the gene encoding Blimp-1 (Prdm1) and Id3 after infection of the recipient mice with a recombinant strain of vaccinia virus encoding the cognate antigen gp100 (gp100-VV). Prdm1 expression was low in naive T cells but progressively increased after infection, and was inversely related to Id3 expression (Fig. 1a). We found a similar expression pattern for Blimp-1 and Id3 protein in naive and activated CD8+ T cells (Fig. 1b). We observed in late effector T cells a decrease in Blimp-1 that was not paralleled by an increase in Id3 (Fig. 1b), which indicated that once repressed, Id3 expression cannot be regained by the sole removal of Blimp-1. We further confirmed high expression of Prdm1 mRNA in SLECs isolated ex vivo, whereas MPECs showed enrichment for Id3 (Fig. 1c). We observed the same expression pattern for Blimp-1 and Id3 protein (Fig. 1d). These findings indicated that Blimp-1 and Id3 are inversely regulated and suggested that Blimp-1 might directly repress Id3 expression.
(a) Quantitative RT-PCR analysis of the expression of Prdm1 and Id3 mRNA in naive pmel-1 CD8+ T cells before (0) or 1–6 d after (horizontal axis) adoptive transfer into recipient wild-type mice infected with gp100-VV; results are presented relative to Actb mRNA (encoding β-actin). (b) Immunoblot analysis of Blimp-1 and Id3 in CD8+ T cells 0–8 d (above lanes) after stimulation with antibody to CD3 (anti-CD3), anti-CD28 and IL-2; β-actin serves as a loading control throughout. (c) Quantitative RT-PCR analysis of the expression of Prdm1 and Id3 in KLRG-1+ and KLRG-1− pmel-1 CD8+ T cells sorted 5 d after adoptive transfer into wild-type mice infected with gp100-VV; results are presented relative to Actb mRNA. (d) Immunoblot analysis of Blimp-1 and Id3 in KLRG-1+ and KLRG-1− pmel-1 CD8+ T cells obtained as in c. (e) Amplification of the promoter regions of Id3 and Csf1 (nonspecific control) in chromatin immunoprecipitated from effector CD8+ T cells with anti-Blimp-1 (α-Blimp-1) or in input DNA (Input (far left), control for equal starting material). α-IgG, antibody to immunoglobulin G (nonspecific control); H2O, water only; gDNA, genomic DNA. (f) Quantitative PCR analysis of the promoter regions of Id3 and Csf1 in chromatin immunoprecipitated with anti-IgG or anti-Blimp-1. NS, not significant; *P < 0.05 and **P < 0.001 (two-tailed t-test). Data are representative of two (a,c–f) or four (b) independent experiments (error bars (c,f), s.e.m. of three samples).
The promoter region of Id3 contains four consensus Blimp-1-binding sites with the sequence AGGGAAAGGG, and it has been shown to be a direct target of Blimp-1 in a human B cell line6,15. To determine whether Blimp-1 directly binds the Id3 promoter in CD8+ T cells, we immunoprecipitated Blimp-1–DNA complexes in effector T cells and amplified the DNA with primers specific for the promoter region of Id3 or the gene encoding colony-stimulating factor 1 (Csf1), which is not targeted by Blimp-1 (ref. 15). We found that Id3 was specifically precipitated in effector T cells but Csf1 was not (Fig. 1e). We further confirmed those results by chromatin immunoprecipitation followed by quantitative PCR, which showed significant enrichment for Blimp-1 in the Id3 promoter region but not in the Csf1 promoter region (Fig. 1f). Together these findings indicated that Id3 was directly targeted and repressed by Blimp-1 in effector CD8+ T lymphocytes.
Id3 is essential for CD8+ memory T cell formation
The function of Id3 has been extensively characterized in thymocyte development17, but its role in mature T cells has only just begun to be addressed18. Id3−/− mice have defective thymocyte development due to impairment in positive and negative selection17, which thereby inexorably compromises the mature T cell compartment19. Consistent with published observations19, we found that Id3−/− CD8+ T cells as well as pmel-1 Id3−/− CD8+ T cells had an activated phenotype characterized by upregulation of CD44 and downregulation of CD62L (Supplementary Fig. 1a). The memory-like phenotype of Id3−/− CD8+ T cells might have been dependent on IL-4 released by a dysregulated Id3−/− natural killer T cell population19, as well as the combined effects of homeostatic proliferation and possible expansion of autoreactive clonotype populations that were not deleted during negative selection (Supplementary Fig. 1b,c). Indeed, the phenotype of pmel-1 Id3−/− T cell was completely restored by removal of Id3−/− natural killer T cells and endogenous T cell antigen receptor α- and β-chains and normalization of T cell homeostasis in mixed–bone marrow chimeras generated by the transfer of bone marrow from pmel-1 mice deficient in recombination-activating gene 1 (pmel-1 Rag1−/− mice) and pmel-1 Rag1−/−Id3−/− mice into wild-type host mice (Supplementary Fig. 1d,e). Thus, to study the role of Id3 in a CD8+ T cell immune response, we used naive CD44−CD62L+ CD8+ T cells isolated from pmel-1 Rag1−/−Id3−/− mice (Fig. 2a).
(a) Flow cytometry of pmel-1 Rag1−/−Id3+/+ and pmel-1 Rag1−/−Id3−/− T cells before (left) and after (Post-sort; right) sorting of naive CD8+ T cells, after gating on CD8+ cells. Numbers in bottom right quadrants indicate percent CD44−CD62L+ cells. (b) Flow cytometry analysis of the incorporation of BrdU into splenocytes 4 d after adoptive transfer of pmel-1 Rag1−/−Id3+/+ or pmel-1 Rag1−/−Id3−/− CD8+ T cells into wild-type mice infected with gp100-VV, followed by treatment of recipient mice with 1.5 mg BrdU 16 h before analysis. Numbers above bracketed lines indicate percent BrdU+ cells. (c) Flow cytometry of the expression of Ly5.2 by pmel-1 CD8+ T cells after adoptive transfer of 6 × 103 pmel-1 Rag1−/− Id3+/+ or pmel-1 Rag1−/−Id3−/− CD8+ T cells into wild-type mice infected with gp100-VV, assessed 0–32 d after infection (above plots). Numbers adjacent to outlined areas indicate percent Ly5.2+ T cells after gating on CD8+ cells. SSC, side scatter. (d) Quantification of Ly5.2+ pmel-1 CD8+ T cells in c, assessed 0–35 d after infection (horizontal axis). (e) Frequency of CD62L+ or KLRG-1+ pmel-1 Rag1−/− Id3+/+ or pmel-1 Rag1−/−Id3−/− CD8+ T cells after transfer into wild-type mice infected with gp100-VV, assessed 6 d after infection. (f) Ly5.2+ pmel-1 CD8+ T cells in the spleen, lymph nodes (LN) and lungs after adoptive transfer of 4 × 104 pmel-1 Rag1−/−Id3+/+ or pmel-1 Rag1−/−Id3−/− CD8+ T cell into wild-type mice infected with gp100-VV, assessed 30 d after infection. Each symbol represents an individual mouse; small horizontal lines indicate the mean (and s.e.m.). *P < 0.05, **P < 0.01 and ***P < 0.001 (two-tailed t-test). Data are representative of two independent experiments (error bars (d,e), s.e.m. of three to four samples).
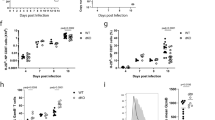
Id3 has been shown to regulate the proliferation, differentiation and survival of cells, depending on the cell type20. We therefore measured the population expansion, differentiation and long-term persistence of adoptively transferred naive pmel-1 Rag1−/−Id3−/− and pmel-1 Rag1−/−Id3+/+ T cells after infection of recipient mice with gp100-VV. To evaluate T cell proliferation, we injected the thymidine analog BrdU 16 h before analyzing its uptake in splenocytes. We found that pmel-1 Rag1−/−Id3−/− T cells and pmel-1 Rag1−/−Id3+/+ control T cells proliferated similarly (58% ± 2.7% Brdu+ Id3−/− T cells versus 61% ± 2.7% Brdu+ Id3+/+ T cells (± s.e.m.); Fig. 2b), and both populations expanded almost 100-fold in the spleen at the peak of the immune response 5 d after infection (Fig. 2c,d), which indicated that in mature CD8+ T cells, Id3 is dispensable for proliferation. Furthermore, we found no difference in the composition of pmel-1 Rag1−/−Id3−/− and pmel-1 Rag1−/−Id3+/+ effector T cell subsets, as assessed by the expression of CD62L and KLRG-1 (Fig. 2e); this emphasized that Id3 also does not control T cell differentiation in response to antigenic stimulation. However, there was a significant difference in the survival of the effector T cell population, which contracted substantially in the absence of Id3 and failed to form physiological numbers of memory T cells (Fig. 2c,d and Supplementary Fig. 2). These results were not due to differences in organ tropism because we found fewer pmel-1 Rag1−/−Id3−/− CD8+ memory T cells in both lymphoid and nonlymphoid tissues (Fig. 2f). In contrast, Id3, which has high expression in naive T cells, was dispensable for their long-term survival (Supplementary Fig. 3). These findings indicated that Id3 is essential for the formation of CD8+ memory T cells and suggested that downregulation of Id3 by Blimp-1 is a programmed switch that controls the survival of effector T cell populations and their maturation into long-lived memory T cells.
Enforced expression of Id3 promotes SLEC persistence
Given the results described above, we hypothesized that Blimp-1 limits the long-term survival of effector T cells by repressing Id3 transcription. We therefore sought to determine whether constitutive expression of Id3 enhanced the generation of memory CD8+ T cells. We transduced pmel-1 CD8+ T cells with a multicistronic retrovirus based on the self-cleaving 2A peptide21 encoding V5-tagged Id3 and Thy-1.1 (Id3–Thy-1.1) or Thy-1.1 alone (Supplementary Fig. 4a). Expression of Thy-1.1 indicated a similarly high transduction efficiency of the two constructs (Fig. 3a). V5-tagged Id3 was detected by immunoblot analysis with Id3-specific antibody as a band with slightly slower migration than endogenous Id3 protein (Fig. 3b) or by V5-specific antibody (Supplementary Fig. 4b). We evaluated the proliferative response and persistence of adoptively transferred Id3–Thy-1.1+ pmel-1 CD8+ T cells after infection of recipient mice with gp100-VV. We observed no difference in the phenotype or function of cells transduced to express Thy-1.1 and those transduced to express Id3–Thy-1.1 before transfer (Supplementary Fig. 5). The proliferation of Id3-overexpressing CD8+ T cells was similar to that of control Thy-1.1+ cells (Fig. 3c,d), which indicated that enforced expression of Id3 did not have a substantial effect on the proliferative ability of developing effector T cells. However, we observed a notable difference between these two groups in long-term survival. Whereas about 90% of control Thy-1.1+ CD8+ T cells underwent physiological population contraction after the peak of the effector response, large numbers of Id3–Thy-1.1+ pmel-1 CD8+ T cells persisted (Supplementary Fig. 6), which resulted in tenfold more persisting memory T cells in the spleen (Fig. 3c,d). Thus, experiments of gain and loss of function led to the same conclusion, that Id3 is a key regulator of memory CD8+ T cell formation.
(a) Frequency of Thy-1.1+ cells (numbers above bracketed lines) among pmel-1 CD8+ T cells transduced with retrovirus expressing Id3–Thy-1.1 or Thy-1.1 alone. (b) Immunoblot analysis of pmel-1 CD8+ T cells transduced as in a, probed with anti-Id3. (c) Flow cytometry of splenic T cells after adoptive transfer of 6 × 102 pmel-1 CD8+ T cells (transduced as in a) into wild-type mice infected with gp100-VV, assessed 4–34 d after infection (above plots). Numbers adjacent to outlined areas indicate percent Thy-1.1+ T cells after gating on CD8+ cells. (d) Abundance of Thy-1.1+ pmel-1 CD8+ T cells in the spleen after adoptive transfer of pmel-1 CD8+ T cells (transduced as in a) into wild-type mice infected with gp100-VV, assessed 0–35 d after infection (horizontal axis). (e,f). Frequency of KLRG-1+ (e) or CD62L+ (f) T cells among Thy-1.1+ pmel-1 CD8+ T cells as in d. (g,h) Frequency of Thy-1.1+ pmel-1 CD8+ T cells producing IFN-γ or IL-2 after stimulation with a leukocyte-activation 'cocktail', assessed 5 d (g) or 30 d (h) after adoptive transfer into wild-type mice infected with gp100-VV. *P < 0.05; **P < 0.01 and ***P < 0.001 (two-tailed t-test). Data are representative of five (a,c,d), three (b,e,f) or two (g,h) independent experiments (error bars (d–h), s.e.m. of three to four samples).
Prdm1−/− CD8+ T cells have high expression of Id3 and 'preferentially' form MPECs rather than SLECs, which results in more memory T cells5,6,7. We therefore hypothesized that Id3 overexpression would mimic Blimp-1 deficiency and result in enrichment for MPECs. Contrary to our assumption, we found no difference between Id3-overexpressing CD8+ T cell populations and Thy-1.1+ control cell populations in their frequency of KLRG-1+ or CD62L+ CD8+ T cells at the peak of the effector response (Fig. 3e,f). Furthermore, intracellular cytokine staining showed similar secretion profiles for interferon-γ (IFN-γ) and IL-2 at 5 d after infection (Fig. 3g), which indicated that effector T cells generated under constitutive expression of Id3 were also functionally similar to Thy-1.1+ control cells. However, whereas most SLECs in the control group underwent apoptosis after the peak of the immune response and continued to decrease in abundance, Id3-overexpressing KLRG-1+ T cells persisted in great abundance over time (Fig. 3e). This difference in the survival of Id3–Thy-1.1+ pmel-1 CD8+ T cells and Thy-1.1+ control cells resulted in enrichment for effector memory T cells 1 month after infection, as manifested by the low frequency of CD62L+ T cells (Fig. 3f) and IL-2-producing T cells (Fig. 3h) at this time point.
Because enforced expression of Id3 prevented contraction of the SLEC population, which enhanced the accumulation of effector memory T cells, we reasoned that peripheral tissues might be further enriched for Id3-overexpressing memory T cells relative to their abundance in lymph nodes, which are niches for central memory T cells. Consistent with our hypothesis, we observed a significantly higher frequency of Id3–Thy-1.1+ pmel-1 CD8+ T cells than Thy-1.1+ control cells in the lungs but not in the lymph nodes as result of 'preferential' accrual of CD62L−KLRG-1+ cells in the periphery (Fig. 4). Together these results showed that Id3 alone was sufficient to rescue SLECs from apoptosis and indicated that Blimp-1, which has high expression in KLRG-1+ T cells, controlled their survival by regulating Id3 expression.
(a) Flow cytometry of T cells from spleen, lymph nodes and lungs after adoptive transfer of 3 × 105 pmel-1 CD8+ T cells (transduced as in Fig. 3a to express Thy-1.1 or Id3–Thy-1.1) into wild-type mice infected with gp100-VV, assessed 40 d after infection. Numbers adjacent to outlined areas indicate percent Thy-1.1+ T cells after gating on CD8+ cells. (b) Frequency of Thy-1.1+ pmel-1 CD8+ T cells in the spleen, lymph nodes and lungs of mice treated as in a. (c) Flow cytometry of Thy-1.1+ pmel-1 CD8+ T cells from the spleen, lymph nodes and lungs of mice treated as in a. Numbers in quadrants indicate percent KLRG-1+CD62L− cells (top left) or KLRG-1−CD62L+ cells (bottom right) after gating on Thy-1.1+ CD8+ cells. *P < 0.05 and **P < 0.01 (two-tailed t-test). Data are representative of two independent experiments (error bars (b), s.e.m. of three samples).
Ectopic expression of Id3 enhances recall responses
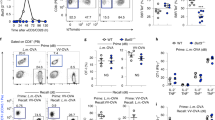
A key attribute of memory T cells is the ability to proliferate and differentiate into large numbers of effector T cells in response to a secondary antigenic challenge. We therefore sought to determine whether the larger pool of memory CD8+ T cells generated under constitutive expression of Id3 would result in superior recall responses. At 30 d after adoptive transfer of Id3-overexpressing CD8+ T cells into wild-type mice infected with gp100-VV, we challenged the hosts with a heterologous fowlpox virus encoding gp100 and evaluated T cell population expansion and secondary memory formation. We observed a threefold increase in pmel-1 CD8+ T cells at the peak of the secondary effector phase in mice that received Id3-overexpressing T cells (Fig. 5a,b). Despite such a relatively small difference in the magnitude of the effector response, we again observed a survival advantage for Id3-overexpressing effector T cells that led to more secondary memory T cells, although these differences were not as pronounced as those in primary responses (Fig. 5a,b). These results indicated that Id3 was sufficient to restore the survival but not the proliferative ability of SLECs.
(a) Flow cytometry of splenic T cells after adoptive transfer of 6 × 102 pmel-1 CD8+ T cells (transduced as in Fig. 3a to express Thy-1.1 or Id3–Thy-1.1) into wild-type mice infected with gp100-VV, followed by secondary challenge with gp100 fowlpox virus 30 d after primary infection, assessed 3–30 d after secondary infection (above plots). Numbers adjacent to outlined areas indicate percent Thy-1.1+ T cells after gating on CD8+ cells. (b) Abundance of Thy-1.1+ pmel-1 CD8+ T cells in the spleen, assessed 0–35 d after secondary infection as described in a (horizontal axis). (c) Survival of wild-type mice challenged with 1 × 105 B16 melanoma cells 30 d after vaccination with gp100-VV with no adoptive transfer (No CD8+ T cells) or after adoptive transfer of pmel-1 CD8+ T cells transduced (as in Fig. 3a) to express Id3–Thy-1.1 or Thy-1.1. *P < 0.05 and **P < 0.001 (log-rank (Mantel-Cox) test). Data are representative of (a,b) or pooled from (c) two independent experiments (error bars (b), s.e.m. of three to seven samples).
To determine whether memory CD8+ T cells generated under constitutive expression of Id3 would confer greater protection, we assessed their ability to reject challenge by gp100+ B16 melanoma. B16 melanoma cells are poorly immunogenic, as manifested by the inability of pmel-1 mice to delay tumor growth relative to its growth in wild-type mice16. The addition of Thy-1.1-transduced pmel-1 memory CD8+ T cells did not improve the survival of tumor-challenged mice relative to that of mice infected with gp100-VV alone (Fig. 5c). In contrast, transfer of pmel-1 memory T cells constitutively expressing Id3 significantly enhanced the survival of mice challenged with B16 cells (Fig. 5c). Together these findings indicated that Id3 overexpression improved secondary memory responses by increasing the absolute number of effector memory T cells.
Id3 induces memory T cell formation independently of Id2
The Id family member Id2 has been reported to affect the magnitude of CD8+ effector responses and, consequently, the generation of long-lived memory T cells22. In contrast to Id3 mRNA, which was progressively downregulated in differentiating effector T cells, Id2 mRNA increased, reaching maximal expression at the peak of the effector response22 (Figs. 1a and 6a). In addition, we found that Id2 and Id3 also had a reciprocal expression pattern in effector T cell subsets, with enrichment for Id2 in SLECs and enrichment for Id3 in MPECs (Figs. 1c,d and 6b). To determine whether Id2 expression was affected by Id3, we assessed the Id2 transcripts in pmel-1 Rag1−/−Id3−/− or Id3–Thy-1.1+ effector cells relative to their abundance in pmel-1 Rag1−/−Id3+/+ and Thy-1.1+ control T cells, respectively. Neither deletion nor enforced expression of Id3 altered the expression of Id2 in effector cells (Fig. 6c), a result consistent with the finding that Id2 expression is similar in Id3−/− and Id3+/+ splenocytes, as assessed by RNA blot23. These results indicated that Id2 was not redundant with Id3 nor did it contribute to the function of Id3 as a regulator of memory CD8+ T cell formation.
(a) Quantitative RT-PCR analysis of Id2 mRNA in naive pmel-1 CD8+ T cells, before (0) or 1–6 d after (horizontal axis) adoptive transfer into wild-type recipient mice infected with gp100-VV; results are presented relative to Actb mRNA. (b) Immunoblot analysis of Id2 in KLRG-1+ or KLRG-1− pmel-1 CD8+ T cells sorted 5 d after adoptive transfer into wild-type mice infected with gp100-VV. (c) Quantitative RT-PCR analysis of Id2 mRNA in pmel-1 Rag1−/−Id3+/+ and pmel-1 Rag1−/−Id3−/− CD8+ T cells (left) or pmel-1 CD8+ T cells transduced (as in Fig. 3a) to express Id3–Thy-1.1 or Thy-1.1 (right), assessed 5 d after adoptive transfer into wild-type mice infected with gp100-VV; results are presented relative to Actb mRNA. Data are representative of two independent experiments (error bars (c), s.e.m. of triplicates).
Id3 induces genes that regulate DNA replication and repair
To better understand the mechanisms by which Id3 regulates CD8+ T cell survival and memory formation, we compared the transcriptomes of pmel-1 Rag1−/−Id3−/− and pmel-1 Rag1−/−Id3+/+ CD8+ T cells as well as Thy-1.1+ and Id3–Thy-1.1+ CD8+ T cells sorted ex vivo 5 d after infection gp100-VV (Fig. 7a,b and Supplementary Table 1). We found 138 genes affected by the gain or loss of function of Id3 (>1.3-fold change in expression and false-discovery rate of P < 0.05; Fig. 7a,b and Supplementary Table 1). To accurately assess the amount of functional Id3, we excluded from the analysis Id3 probe sets specific for transcript segments other than those affected by transgenic manipulation (Supplementary Fig. 7). Id3 was the gene most significantly affected, with 3.8-fold higher expression in pmel-1 Rag1−/−Id3+/+ CD8+ T cells than in pmel-1 Rag1−/−Id3−/− cells (P = 9.3 × 10−9) and 42.9-fold higher expression in Id3–Thy-1.1+ CD8+ T cells than in Thy-1.1+ control T cells (P = 5.6 × 10−14); this confirmed the integrity of these assays. We observed similar transcriptional differences between pmel-1 Rag1−/−Id3−/− cells and pmel-1 Rag1−/−Id3+/+ cells and between Thy-1.1+ cells and Id3–Thy-1.1+ T cells, as shown by heat maps of their expression patterns (Fig. 7a,b). About one-third of the genes encoded molecules involved in DNA replication and repair (Supplementary Table 1), many of which could be visualized in a defined network (Supplementary Fig. 8). Almost all genes encoding molecules that regulate DNA replication and repair were reciprocally modulated by Id3 manipulation, such that genes downregulated in pmel-1 Rag1−/−Id3−/− T cells were induced by Id3 overexpression and vice versa (Fig. 7c and Supplementary Table 1). These genes included Mcm2, Mcm3 and Mcm10 (which encode components of the minichromosome maintenance complex); Top2a (which encodes topoisomerase (DNA) IIα); Kif2c, Kif4a, Kif14 and Kif20a (which encode members of the kinesin family); Cdca5 and Cdca8 (which encode proteins associated with the cell division cycle); Foxm1 (which encodes the transcription factor Foxm1); and Nek2 (which encodes the serine-threonine kinase Nek2). The molecules encoded either are 'caretakers' essential to the maintenance of genome integrity during cell division or have crucial roles in ensuring proper chromosome segregation during mitosis and fidelity of the cell-division process24,25,26,27,28,29,30,31. Dysregulation of these molecules results in serious aberrancies during mitosis, such as chromosome mis-segregation, cytokinesis defects and overt aneuploidy, which can trigger apoptosis32. The gene encoding the serine-threonine kinase Stk39, which mediates apoptosis after genotoxic stress33, was downregulated by Id3, which further emphasized the importance of Id3 in regulating genes essential for genome integrity.
(a,b) Heat map of genes with different expression in pmel-1 Rag1−/−Id3+/+ or pmel-1 Rag1−/−Id3−/− CD8+ T cells (a) or pmel-1 CD8+ T cells transduced (as in Fig. 3a) to express Id3–Thy-1.1 or Thy-1.1 (b), sorted 5 d after adoptive transfer into wild-type mice infected with gp100-VV (>1.3-fold change in expression; false discovery rate, P < 0.05). Brackets (top) indicate hierarchical clustering. (c) Change in the expression of genes encoding molecules involved in the DNA-replication and DNA-repair network, showing expression in pmel-1 Rag1−/−Id3−/− CD8+ T cells or Id3–Thy-1.1+ pmel-1 CD8+ T cells relative to expression in pmel-1 Rag1−/−Id3+/+ or Thy-1.1+ control T cells, respectively. Data represent one experiment each with quadruplicates (a,b) or the combined analysis of two independent experiments (c; error bars, s.e.m.).
It is noteworthy that the pro- and antiapoptotic factors linked to the Id2-mediated survival of effector T cells, including Bcl-2, Serpinb9 and Bcl-2l11, were not influenced by gain or loss of Id3 function (<1.3-fold change in expression; false-discovery rate, P > 0.05), except the survival factor CTLA-4 (ref. 22). These findings indicated that the mechanisms underlying the prosurvival effects of Id3 and Id2 are distinct. Although Id2 relies on the induction of prototypical antiapoptotic factors, Id3 promotes the survival of CD8+ effector T cells through the coordinated expression of key regulators for genomic stability.
Deletion of E2A enhances CD8+ memory T cell formation
To further explore the mechanism behind the Id3-mediated enhancement of the survival and memory formation of CD8+ T cells, we did gene-set enrichment analysis of our microarray data with the C2 collection of curated gene sets in the Molecular Signatures Database. Notably, a gene set representing transcriptional differences in pre-B-cell lines deficient in Tcf3, which encodes E2A, was mostly absent from pmel-1 Rag1−/−Id3−/− CD8+ T cells in contrast to its presence in pmel-1 Rag1−/−Id3+/+ CD8+ T cells, whereas we observed positive enrichment in Id3–Thy-1.1+ CD8+ T cells relative to its presence in Thy-1.1+ control T cells34,35 (Fig. 8a and Supplementary Fig. 9). Id proteins prevent E proteins from binding to the promoter of target genes by forming heterodimers through helix-loop-helix domains to either repress or activate gene transcription depending on the cellular context17,36,37. The finding that genes with high expression in Tcf3-deficient cells also showed enrichment in T cells with more Id3 suggested that Id3 might act in part through inhibition of E2A transcriptional activity in mature CD8+ T cells. To test whether overexpression of Id3 would impair the DNA-binding ability of E2A in mature T cells, we assessed by electrophoretic mobility-shift assay the ability of nuclear extracts of Id3–Thy-1.1+ effector T cells to interact with oligonucleotides containing the consensus E-box sequence. We found that the intensity of the E-protein DNA-binding band was much lower in T cells constitutively expressing Id3 than in Thy-1.1+ control cells and was attenuated by an E2A-specific antibody (Fig. 8b), which indicated that Id3 antagonized the binding of E2A to DNA in CD8+ effector T cells.
(a) Gene-set enrichment analysis of genes overexpressed in Tcf3-deficient pre-B-cell lines relative to transcriptomes of pmel-1 Rag1−/−Id3−/− or pmel-1 Rag1−/−Id3+/+ CD8+ T cells (left) or pmel-1 CD8+ T cells transduced (as in Fig. 3a) to express Id3–Thy-1.1 or Thy-1.1 (right), sorted 5 d after adoptive transfer into wild-type mice infected with gp100-VV. Enrich, enrichment profile; Hits, genes in functional set; RMS, ranking metric score. (b) Electrophoretic mobility-shift assay of nuclear extracts of pmel-1 CD8+ T cells transduced (as in Fig. 3a) to express Thy-1.1 or Id3–Thy-1.1, incubated with biotin-labeled oligonucleotide probes containing E-box-binding sites (uE5) and/or unlabeled oligonucleotide probes (competitors) and probed with anti-E2A (α-E2A) or nonspecific antibody (Ab) to determine the specificity of shifted bands. (c) Abundance of Ly5.2+ pmel-1 CD8+ T cells in the spleen after adoptive transfer of 6 × 103 pmel-1 Tcf3flox/flox Cre-ERT2 CD8+ T cells (previously activated in vitro for 5 d with (TAM) or without (No TAM) tamoxifen, for the deletion of Tcf3) into wild-type mice infected with gp100-VV. *P < 0.05 and **P < 0.01 (one-tailed t-test). Data represent two separate experiments (left and right) with quadruplicate samples (a) or are representative of six (b) or two (c) independent experiments (error bars (c), s.e.m. of three or four samples).
We next sought to ascertain whether deletion of Tcf3 in CD8+ T cells would reproduce the effect of Id3 overexpression and enhance their ability to form long-lived memory T cells. For these studies we used pmel-1 cells with loxP-flanked Tcf3 alleles and expression of a fusion of Cre recombinase and the estrogen receptor T2 moiety, which retains Cre in the cytosol until tamoxifen is administered (pmel-1 Tcf3flox/floxCre-ERT2 cells). We activated pmel-1 Tcf3flox/floxCre-ERT2 CD8+ T cells in vitro for 5 d with or without tamoxifen to conditionally delete Tcf3 (ref. 38; Supplementary Fig. 10), then adoptively transferred the cells into wild-type mice infected with gp100-VV. Deletion of Tcf3 did not affect the ability of CD8+ T cell populations to expand after antigenic stimulation but significantly enhanced their long-term survival (Fig. 8c), which recapitulated the biology of constitutive expression of Id3. These data suggested that the inhibition of E2A transcriptional activity is critical to the prosurvival effect of Id3.
Discussion
In this study we found that Blimp-1 directly targeted the Id3 promoter in effector CD8+ T cells to repress Id3 expression. Less Id3 in effector T cells triggered the apoptosis of these cells, which limited their ability to enter the memory T cell pool. Enforced expression of Id3 in CD8+ T cells prolonged the survival of SLECs and enhanced the formation of effector memory T cells and recall responses. The prosurvival effects of Id3 were mediated partly through the inhibition of E2A transcriptional activity and the upregulation of genes encoding molecules critical for the maintenance of genome stability in rapidly dividing cells.
Studies of mice with conditional knockout of Prdm1 have shown that Blimp-1 negatively regulates the formation of memory CD8+ T cells5,6,7, but the mechanisms that underlie Blimp-1 activity have thus far been poorly characterized. Blimp-1 is thought to impair the generation of memory CD8+ T cells solely by promoting responding T cells to develop into terminally differentiated SLECs at the expense of MPECs6,7. Similar to what has been noted for other mature lymphocytes2, Blimp-1 might promote terminal differentiation by suppressing the transcriptional repressor Bcl-6, which in CD8+ T cells inhibits expression of the gene encoding granzyme B39 and promotes the generation of central memory T cells40. Here we found that in addition to driving T cells toward senescence, Blimp-1 also triggered death in these terminally differentiated cells. This proapoptotic program 'instructed' by repression of Id3 and subsequent increase in E2A transcriptional activity was characterized by the downregulation of many genes encoding molecules necessary for the maintenance of genome integrity in proliferating cells. Those findings are consistent with our observation that Id3 was dispensable for the survival of naive T cells, a relatively quiescent T cell subset.
Furthermore, our findings shed light on the different roles of members of the Id family in mature CD8+ T cells. During immune responses, Id3 and its homolog Id2 show an inverse pattern of expression during effector differentiation in response to infection22. Expression of Id2 is required for the survival of effector CD8+ T cells during the expansion phase and therefore controls the magnitude of the effector response22. However, Id2 deficiency does not affect the fraction of T cell undergoing apoptosis after the peak of the response, and the larger number of wild-type memory T cells than Id2−/− memory T cells is a direct reflection of the extent of effector T cell population expansion22. Those findings, together with our results here, indicate that similar to their actions in other cell types20, the regulation and function of Id2 and Id3 are nonredundant in mature CD8+ T cells. Id2 seems to be critical for the short-term survival of effector T cells but it does not affect their population contraction and long-term persistence. Conversely, Id3, which shows enrichment in MPECs, is required for their long-term survival and entry into the memory pool but does not affect the magnitude of the effector T cell response.
Considerable effort has been devoted to the induction of robust CD8+ memory T cell responses for the prevention and treatment of intracellular pathogens and cancer. Through constitutive expression of Id3, we were able to prolong the survival of SLECs and generate up to 20-fold more memory T cells than were generated by control cells transduced to express Thy-1.1 alone. Although a substantially greater frequency of CD8+ memory T cells prolonged the survival of hosts challenged with B16 tumor, it did not completely protect mice from tumor uptake. Those data indicated that the mere larger number of memory T cells alone was insufficient for effective protection and emphasize the importance of qualitative aspects of memory T cells41,42. Furthermore, our findings suggest that heterologous boost vaccination strategies that augment the frequency of memory T cells but also drive cells toward terminal differentiation43 would have limited preventative and therapeutic success. Pharmacological modulation of Wnt–β-catenin signaling pathways44,45,46 and kinase mTOR pathways47,48, which are emerging as critical regulators of central memory T cells and CD8+ memory stem cells, might be a more effective strategy for generating qualitatively better memory T cells49.
Methods
Mice and tumor lines.
C57BL/6 mice, Ly5.1 (B6.SJL-PtprcaPepcb/BoyJ) mice, Id3−/− (B6.129S-Id3tm1Zhu/J) mice, Tcf3flox/flox (C57BL/6; 129S4-Tcf3tm4Zhu/J) mice, Rag 1−/− (C57BL/6; 129S7-Rag1tm1Mom/J) mice and pmel-1 (B6.Cg-Thy1a/Cy Tg(TcraTcrb)8Rest/J) mice were from the Jackson Laboratory; Cre-ERT2 (B6-Gt(ROSA)26Sortm9(cre/Esr1)Arte) mice were from Taconic. The pmel-1 mice were crossed with Rag1−/− mice and Id3−/− mice for the generation of pmel-1 Rag1−/−Id3−/− mice and were crossed with Tcf3flox/flox mice and Cre-ERT2 mice for the generation of pmel-1 Tcf3flox/floxCre-ERT2 mice. B16 (H-2Db), a gp100+ mouse melanoma, and MCA205 (H-2Db), a gp100− sarcoma, were from the National Cancer Institute Tumor Repository. All mouse experiments were done with the approval of the National Cancer Institute Animal Use and Care Committee.
Antibodies, flow cytometry and cell sorting.
Anti-BrdU (3D4), anti-Ly5.2 (104), anti-Thy-1.1 (OX-7), anti-CD44 (IM7), anti-CD62L (MEL-14), anti-CD8α (53-6.7), anti-KLRG-1 (2F1), anti-IL-2 (JE56-5H4) and anti-IFN-γ (XMG1.2) were from BD Biosciences. Leukocyte Activation Cocktail containing phorbol myristate acetate and ionomycin (BD Biosciences) was used for the stimulation of T cells for intracellular cytokine staining. A FACSCanto I or FACSCanto II (BD Biosciences) was used for flow cytometry acquisition. Samples were analyzed with FlowJo software (TreeStar). Naive CD8+ T cells were sorted with a FACSAria (BD Biosciences).
Real-time RT-PCR.
RNA was isolated with an RNeasy Mini kit (Qiagen) and cDNA was generated by reverse transcription (Applied Biosystems). Primers from Applied Biosystems and a Prism 7900HT (Applied Biosystems) were used for real-time PCR analysis of all genes; results are presented relative to Actb expression.
Immunoblot analysis.
Proteins were separated by 4–12% SDS-PAGE, followed by standard immunoblot analysis with anti-Id2 (9-2-8; CalBioreagents), anti-Id3 (6-1; CalBioreagents), anti-Blimp-1 (6D3; eBioscience), anti-β-actin (C4; Santa Cruz Biotechnology), horseradish peroxidase–conjugated goat anti–mouse IgG (sc-2031; Santa Cruz Biotechnology) and horseradish peroxidase–conjugated goat anti–rabbit IgG (sc-2030; Santa Cruz Biotechnology).
Chromatin immunoprecipitation.
A Millipore 17–295 ChIP kit was used for chromatin immunoprecipitation. DNA-protein complexes were crosslinked with formaldehyde at a final concentration of 1%, followed by precipitation with nonspecific anti-IgG (12-371; Millipore) or chromatin immunoprecipitation–grade anti-Blimp-1 (6D3; eBioscience). The promoter region adjacent to the transcription start site of Id3 (primers, 5′-GGTCCATGCTTTTTCTTTCTCCGTGGAAAAGG-3′ and 5′-GGGAAAAAATTAATTGCGGTGAAGCTGAGG-3′) or Csf1 (primers, 5′-AGCTGGATGCTCCCCACTTCTCCCTACAG-3′ and 5′-CAGGACTTGAATGGGGATGGACCAACG-3′) was amplified from the purified DNA-protein complex.
Retroviral vector construction and virus production.
Id3 cDNA plus sequence encoding the V5 tag and Thy-1.1 linked by sequence encoding the picornavirus 2A ribosomal skip peptide21 was cloned together into the MSGV-1 vector. Platinum Eco cell lines (Cell Biolabs) were used for gamma-retroviral production by transfection with DNA plasmids through the use of Lipofectamine 2000 (Invitrogen) and collection of virus 40 h after transfection.
In vitro activation and transduction of CD8+ T cells.
CD8+ T cells were separated from non-CD8+ T cells with a MACS negative selection kit (Miltenyi Biotech) and were activated on plates coated with anti-CD3ɛ (2 μg/ml; 145-2C11; BD Biosciences) and soluble anti-CD28 (1 μg/ml; 37.51; BD Biosciences) in culture medium containing IL-2 (10 ng/ml; Chiron). Virus was 'spin-inoculated' at 2,000g for 2 h at 32 °C onto plates coated with retronectin (Takara). CD8+ T cells activated for 24 h were spun onto plates after aspiration of viral supernatants.
Adoptive cell transfer, infection and tumor challenge.
Adoptive transfer of cells (6 × 102 to 3 × 105 cells) and infection with recombinant vaccinia virus or fowlpox virus expressing human gp100 (rFPhgp100; Therion Biologics) were done as described16. Female C57BL/6 mice were injected subcutaneously with 1 × 105 B16 melanoma cells.
Counting of adoptively transferred cells.
Mice were killed after infection. Samples were enriched for CD8+ T cells (MACS negative selection kit) and cells were counted by trypan blue exclusion. The frequency of transferred T cells was determined by measurement of the expression of CD8 and Thy-1.1, Ly5.2 or CFSE (carboxyfluorescein succinimidyl ester) by flow cytometry. The absolute number of pmel-1 cells was calculated by multiplication of the total cell count with the percentage of CD8+Thy-1.1+, CD8+Ly5.2+ or CD8+CFSE+ cells.
Microarray analysis.
Total RNA was isolated (with an RNEasy Micro kit (Qiagen)) from 1 × 106 pmel-1 Rag1−/−Id3−/− or pmel-1 Rag1−/−Id3+/+ CD8+ T cells and 3 × 105 pmel-1 CD8+ T cells transduced to express Id3–Thy-1.1 or Thy-1.1, sorted 5 d after adoptive transfer into wild-type mice infected with gp100-VV, then was processed with a WT Expression kit (Ambion), fragmented and labeled with a WT Terminal Labeling Kit (Affymetrix), hybridized to a Mouse Gene 1.0 ST array (Affymetrix) and stained on a GeneChip Fluidics Station 450 (Affymetrix). Microarrays were scanned on a GeneChip Scanner 3000 7G (Affymetrix). Raw data from the generated cell-intensity files ('.cel' extension) were imported into Partek Genomics Suite by the robust multiarray average method. Data at the level of the probe set were merged into data sets at the level of genes on the basis of the median probe-set signal. For accurate assessment of the amount of functional Id3 mRNA in engineered CD8+ T cells, Id3-specific probe sets specific for transcript segments other than those affected by transgenic manipulation were excluded from all analyses. Genes with differences in expression were identified by two-way analysis of variance (Partek) with the incorporation of both the experimental batch and transgenic up- or downregulation of Id3 as factors in the analysis. Genes with differences in expression were filtered by the Benjamini-Hochberg false-discovery rate procedure (P < 0.05) and a between-group 'fold-change' criterion of over 1.3 (P < 0.05). The Ingenuity Pathway Analysis system was used for Pathway Analysis of the lists of genes identified with differences in expression.
Electrophoretic mobility-shift assay.
Nuclear extracts (5 μg protein) isolated with NE-PER Nuclear and Cytoplasmic Extraction Kit (Thermo Scientific) were incubated with oligonucleotide probes labeled with biotin at the 3′ end (sense, 5′-AGCTCCAGAACACCTGCAGCAG-3′; antisense, 5′-CTGCTGCAGGTGTTCTGGAGCT-3′) containing the E protein–binding motif 5′-AACACCTGCA-3′. Unlabeled oligonucleotide probes were used as competitors. Anti-E2A (G127-32; BD Biosciences) and nonspecific IgG (R35-95; BD Biosciences) were used for confirmation of specificity. Samples were separated by electrophoresis through a NativePAGE Novex 3–12% Bis-Tris gel (Invitrogen) and were blotted onto a nylon membrane before exposure to X-ray film.
Statistical analyses.
A one-tailed or two-tailed unpaired t-test was used for comparison of data based on experimental hypothesis. A log-rank test was used for analysis of survival curves.
Additional methods.
Information on the generation of bone marrow chimeras, cytokine release and BrdU- or annexin-incorporation assays is available in the Supplementary Methods.
Accession codes.
GEO: microarray data, GSE23568.
Accession codes
References
Turner, C.A. Jr., Mack, D.H. & Davis, M.M. Blimp-1, a novel zinc finger-containing protein that can drive the maturation of B lymphocytes into immunoglobulin-secreting cells. Cell 77, 297–306 (1994).
Crotty, S., Johnston, R.J. & Schoenberger, S.P. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat. Immunol. 11, 114–120 (2010).
Magnusdottir, E. et al. Epidermal terminal differentiation depends on B lymphocyte-induced maturation protein-1. Proc. Natl. Acad. Sci. USA 104, 14988–14993 (2007).
Nishikawa, K. et al. Blimp1-mediated repression of negative regulators is required for osteoclast differentiation. Proc. Natl. Acad. Sci. USA 107, 3117–3122 (2010).
Shin, H. et al. A role for the transcriptional repressor Blimp-1 in CD8+ T cell exhaustion during chronic viral infection. Immunity 31, 309–320 (2009).
Rutishauser, R.L. et al. Transcriptional repressor Blimp-1 promotes CD8+ T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity 31, 296–308 (2009).
Kallies, A., Xin, A., Belz, G.T. & Nutt, S.L. Blimp-1 transcription factor is required for the differentiation of effector CD8+ T cells and memory responses. Immunity 31, 283–295 (2009).
Joshi, N.S. et al. Inflammation directs memory precursor and short-lived effector CD8+ T cell fates via the graded expression of T-bet transcription factor. Immunity 27, 281–295 (2007).
Sarkar, S. et al. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J. Exp. Med. 205, 625–640 (2008).
Belz, G.T. & Kallies, A. Effector and memory CD8+ T cell differentiation: toward a molecular understanding of fate determination. Curr. Opin. Immunol. 22, 279–285 (2010).
Rutishauser, R.L. & Kaech, S.M. Generating diversity: transcriptional regulation of effector and memory CD8 T-cell differentiation. Immunol. Rev. 235, 219–233 (2010).
Ichii, H. et al. Role for Bcl-6 in the generation and maintenance of memory CD8+ T cells. Nat. Immunol. 3, 558–563 (2002).
Martins, G.A., Cimmino, L., Liao, J., Magnusdottir, E. & Calame, K. Blimp-1 directly represses Il2 and the Il2 activator Fos, attenuating T cell proliferation and survival. J. Exp. Med. 205, 1959–1965 (2008).
Martins, G. & Calame, K. Regulation and functions of Blimp-1 in T and B lymphocytes. Annu. Rev. Immunol. 26, 133–169 (2008).
Shaffer, A.L. et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity 17, 51–62 (2002).
Overwijk, W.W. et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J. Exp. Med. 198, 569–580 (2003).
Rivera, R.R., Johns, C.P., Quan, J., Johnson, R.S. & Murre, C. Thymocyte selection is regulated by the helix-loop-helix inhibitor protein, Id3. Immunity 12, 17–26 (2000).
Maruyama, T. et al. Control of the differentiation of regulatory T cells and TH17 cells by the DNA-binding inhibitor Id3. Nat. Immunol. 12, 86–95 (2010).
Verykokakis, M., Boos, M.D., Bendelac, A. & Kee, B.L. SAP protein-dependent natural killer T-like cells regulate the development of CD8+ T cells with innate lymphocyte characteristics. Immunity 33, 203–215 (2010).
Yokota, Y. Id and development. Oncogene 20, 8290–8298 (2001).
Szymczak, A.L. et al. Correction of multi-gene deficiency in vivo using a single 'self-cleaving' 2A peptide-based retroviral vector. Nat. Biotechnol. 22, 589–594 (2004).
Cannarile, M.A. et al. Transcriptional regulator Id2 mediates CD8+ T cell immunity. Nat. Immunol. 7, 1317–1325 (2006).
Pan, L., Sato, S., Frederick, J.P., Sun, X.H. & Zhuang, Y. Impaired immune responses and B-cell proliferation in mice lacking the Id3 gene. Mol. Cell. Biol. 19, 5969–5980 (1999).
Ibarra, A., Schwob, E. & Mendez, J. Excess MCM proteins protect human cells from replicative stress by licensing backup origins of replication. Proc. Natl. Acad. Sci. USA 105, 8956–8961 (2008).
Jarvinen, T.A. & Liu, E.T. Topoisomerase IIalpha gene (TOP2A) amplification and deletion in cancer–more common than anticipated. Cytopathology 14, 309–313 (2003).
Kinzler, K.W. & Vogelstein, B. Cancer-susceptibility genes. Gatekeepers and caretakers. Nature 386, 761–763 (1997).
Loffler, H., Lukas, J., Bartek, J. & Kramer, A. Structure meets function–centrosomes, genome maintenance and the DNA damage response. Exp. Cell Res. 312, 2633–2640 (2006).
Moynahan, M.E. & Jasin, M. Mitotic homologous recombination maintains genomic stability and suppresses tumorigenesis. Nat. Rev. Mol. Cell Biol. 11, 196–207 (2010).
Warner, S.L., Gray, P.J. & Von Hoff, D.D. Tubulin-associated drug targets: Aurora kinases, Polo-like kinases, and others. Semin. Oncol. 33, 436–448 (2006).
Wonsey, D.R. & Follettie, M.T. Loss of the forkhead transcription factor FoxM1 causes centrosome amplification and mitotic catastrophe. Cancer Res. 65, 5181–5189 (2005).
Xie, S., Xie, B., Lee, M.Y. & Dai, W. Regulation of cell cycle checkpoints by polo-like kinases. Oncogene 24, 277–286 (2005).
Holland, A.J. & Cleveland, D.W. Boveri revisited: chromosomal instability, aneuploidy and tumorigenesis. Nat. Rev. Mol. Cell Biol. 10, 478–487 (2009).
Balatoni, C.E. et al. Epigenetic silencing of Stk39 in B-cell lymphoma inhibits apoptosis from genotoxic stress. Am. J. Pathol. 175, 1653–1661 (2009).
Greenbaum, S., Lazorchak, A.S. & Zhuang, Y. Differential functions for the transcription factor E2A in positive and negative gene regulation in pre-B lymphocytes. J. Biol. Chem. 279, 45028–45035 (2004).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 102, 15545–15550 (2005).
Lin, Y.C. et al. A global network of transcription factors, involving E2A, EBF1 and Foxo1, that orchestrates B cell fate. Nat. Immunol. 11, 635–643 (2010).
Kee, B.L. E. and ID proteins branch out. Nat. Rev. Immunol. 9, 175–184 (2009).
Pan, L., Hanrahan, J., Li, J., Hale, L.P. & Zhuang, Y. An analysis of T cell intrinsic roles of E2A by conditional gene disruption in the thymus. J. Immunol. 168, 3923–3932 (2002).
Yoshida, K. et al. Bcl6 controls granzyme B expression in effector CD8+ T cells. Eur. J. Immunol. 36, 3146–3156 (2006).
Ichii, H., Sakamoto, A., Kuroda, Y. & Tokuhisa, T. Bcl6 acts as an amplifier for the generation and proliferative capacity of central memory CD8+ T cells. J. Immunol. 173, 883–891 (2004).
Klebanoff, C.A., Gattinoni, L. & Restifo, N.P. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol. Rev. 211, 214–224 (2006).
Sallusto, F., Lanzavecchia, A., Araki, K. & Ahmed, R. From vaccines to memory and back. Immunity 33, 451–463 (2010).
Wirth, T.C. et al. Repetitive antigen stimulation induces stepwise transcriptome diversification but preserves a core signature of memory CD8+ T cell differentiation. Immunity 33, 128–140 (2010).
Gattinoni, L. et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat. Med. 15, 808–813 (2009).
Gattinoni, L., Ji, Y. & Restifo, N.P. Wnt/β-catenin signaling in T-cell immunity and cancer immunotherapy. Clin. Cancer Res. 16, 4695–4701 (2010).
Gattinoni, L. et al. A human memory T cell subset with stem cell-like properties. Nat. Med. 17, 1290–1297 (2011).
Araki, K. et al. mTOR regulates memory CD8 T-cell differentiation. Nature 460, 108–112 (2009).
Pearce, E.L. et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460, 103–107 (2009).
Gattinoni, L., Klebanoff, C.A. & Restifo, N.P. Pharmacologic induction of CD8+ T cell memory: better living through chemistry. Sci. Transl. Med. 1, 11ps12 (2009).
Acknowledgements
We thank A. Mixon and S. Farid of the Flow Cytometry Unit for help with flow cytometry analyses and sorting, and M. Bachinski for help with editing the manuscript. Supported by the Intramural Research Program of the National Cancer Institute, Center for Cancer Research of the US National Institutes of Health.
Author information
Authors and Affiliations
Contributions
Y.J., Z.P., M.R., C.A.K., Z.Y., M.S., R.N.R., D.C.P., Z.A.B. and L.G. did experiments; Y.J., Z.P., M.R., C.A.K., E.W. and L.G. analyzed experiments; Y.J., Z.P., P.M., D.S.S., F.M.M., N.P.R. and L.G. designed experiments; and Y.J., N.P.R. and L.G. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10, Table 1 and Methods (PDF 1948 kb)
Rights and permissions
About this article
Cite this article
Ji, Y., Pos, Z., Rao, M. et al. Repression of the DNA-binding inhibitor Id3 by Blimp-1 limits the formation of memory CD8+ T cells. Nat Immunol 12, 1230–1237 (2011). https://doi.org/10.1038/ni.2153
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2153