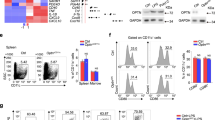
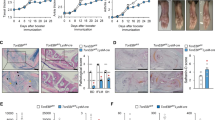
Abstract
Dendritic cells (DCs), which are known to support immune activation during infection, may also regulate immune homeostasis in resting animals. Here we show that mice lacking the ubiquitin-editing molecule A20 specifically in DCs spontaneously showed DC activation and population expansion of activated T cells. Analysis of DC-specific epistasis in compound mice lacking both A20 and the signaling adaptor MyD88 specifically in DCs showed that A20 restricted both MyD88-independent signals, which drive activation of DCs and T cells, and MyD88-dependent signals, which drive population expansion of T cells. In addition, mice lacking A20 specifically in DCs spontaneously developed lymphocyte-dependent colitis, seronegative ankylosing arthritis and enthesitis, conditions stereotypical of human inflammatory bowel disease (IBD). Our findings indicate that DCs need A20 to preserve immune quiescence and suggest that A20-dependent DC functions may underlie IBD and IBD-associated arthritides.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Rakoff-Nahoum, S., Paglino, J., Eslami-Varzaneh, F., Edberg, S. & Medzhitov, R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118, 229–241 (2004).
Kawai, T. & Akira, S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384 (2010).
Chen, G., Shaw, M.H., Kim, Y.G. & Nunez, G. NOD-like receptors: role in innate immunity and inflammatory disease. Annu. Rev. Pathol. 4, 365–398 (2009).
Izcue, A., Coombes, J.L. & Powrie, F. Regulatory lymphocytes and intestinal inflammation. Annu. Rev. Immunol. 27, 313–338 (2009).
Joffre, O., Nolte, M.A., Sporri, R. & Reis e Sousa, C. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol. Rev. 227, 234–247 (2009).
Steinman, R.M. & Banchereau, J. Taking dendritic cells into medicine. Nature 449, 419–426 (2007).
Iwasaki, A. & Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5, 987–995 (2004).
Reis e Sousa, C. Dendritic cells in a mature age. Nat. Rev. Immunol. 6, 476–483 (2006).
Marshak-Rothstein, A. & Rifkin, I.R. Immunologically active autoantigens: the role of toll-like receptors in the development of chronic inflammatory disease. Annu. Rev. Immunol. 25, 419–441 (2007).
Takeuchi, O. & Akira, S. Pattern recognition receptors and inflammation. Cell 140, 805–820 (2010).
Steinman, R.M., Hawiger, D. & Nussenzweig, M.C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21, 685–711 (2003).
Steinman, R.M. & Nussenzweig, M.C. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc. Natl. Acad. Sci. USA 99, 351–358 (2002).
Opipari, A.W. Jr., Boguski, M.S. & Dixit, V.M. The A20 cDNA induced by tumor necrosis factor α encodes a novel type of zinc finger protein. J. Biol. Chem. 265, 14705–14708 (1990).
Lee, E.G. et al. Failure to regulate TNF-induced NF-κB and cell death responses in A20-deficient mice. Science 289, 2350–2354 (2000).
Wertz, I.E. et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-κB signalling. Nature 430, 694–699 (2004).
Boone, D.L. et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat. Immunol. 5, 1052–1060 (2004).
Hitotsumatsu, O. et al. The ubiquitin-editing enzyme A20 restricts nucleotide-binding oligomerization domain containing 2-triggered signals. Immunity 28, 381–390 (2008).
Tavares, R.M. et al. The ubiquitin modifying enzyme A20 restricts B cell survival and prevents autoimmunity. Immunity 33, 181–191 (2010).
Turer, E.E. et al. Homeostatic MyD88-dependent signals cause lethal inflammation in the absence of A20. J. Exp. Med. 205, 451–464 (2008).
Graham, R.R. et al. Genetic variants near TNFAIP3 on 6q23 are associated with systemic lupus erythematosus. Nat. Genet. 40, 1059–1061 (2008).
Musone, S.L. et al. Multiple polymorphisms in the TNFAIP3 region are independently associated with systemic lupus erythematosus. Nat. Genet. 40, 1062–1064 (2008).
Burton, P.R. et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat. Genet. 39, 1329–1337 (2007).
Plenge, R.M. et al. Two independent alleles at 6q23 associated with risk of rheumatoid arthritis. Nat. Genet. 39, 1477–1482 (2007).
Nair, R.P. et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-κB pathways. Nat. Genet. 41, 199–204 (2009).
Elder, J.T. Genome-wide association scan yields new insights into the immunopathogenesis of psoriasis. Genes Immun. 10, 201–209 (2009).
Trynka, G. et al. Coeliac disease-associated risk variants in TNFAIP3 and REL implicate altered NF-κB signalling. Gut 58, 1078–1083 (2009).
Caton, M.L., Smith-Raska, M.R. & Reizis, B. Notch-RBP-J signaling controls the homeostasis of CD8− dendritic cells in the spleen. J. Exp. Med. 204, 1653–1664 (2007).
Birnberg, T. et al. Lack of conventional dendritic cells is compatible with normal development and T cell homeostasis, but causes myeloid proliferative syndrome. Immunity 29, 986–997 (2008).
Ohnmacht, C. et al. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J. Exp. Med. 206, 549–559 (2009).
Hawiger, D. et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 194, 769–779 (2001).
Redmond, W.L. & Sherman, L.A. Peripheral tolerance of CD8 T lymphocytes. Immunity 22, 275–284 (2005).
Luckashenak, N. et al. Constitutive crosspresentation of tissue antigens by dendritic cells controls CD8+ T cell tolerance in vivo. Immunity 28, 521–532 (2008).
Kearney, E.R., Pape, K.A., Loh, D.Y. & Jenkins, M.K. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity 1, 327–339 (1994).
Hou, B., Reizis, B. & DeFranco, A.L. Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms. Immunity 29, 272–282 (2008).
Travis, M.A. et al. Loss of integrin αvβ8 on dendritic cells causes autoimmunity and colitis in mice. Nature 449, 361–365 (2007).
Taurog, J.D. et al. Inflammatory disease in HLA-B27 transgenic rats. Immunol. Rev. 169, 209–223 (1999).
Uhlig, H.H. et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity 25, 309–318 (2006).
Garrett, W.S. et al. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell 131, 33–45 (2007).
The Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447, 661–678 (2007).
D'Agostino, M.A. & Olivieri, I. Enthesitis. Best Pract. Res. Clin. Rheumatol. 20, 473–486 (2006).
Chen, M. et al. Dendritic cell apoptosis in the maintenance of immune tolerance. Science 311, 1160–1164 (2006).
Stranges, P.B. et al. Elimination of antigen-presenting cells and autoreactive T cells by Fas contributes to prevention of autoimmunity. Immunity 26, 629–641 (2007).
Melillo, J.A. et al. Dendritic cell (DC)-specific targeting reveals Stat3 as a negative regulator of DC function. J. Immunol. 184, 2638–2645 (2010).
Manicassamy, S. et al. Activation of β-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science 329, 849–853 (2010).
Song, X.T. et al. A20 is an antigen presentation attenuator, and its inhibition overcomes regulatory T cell-mediated suppression. Nat. Med. 14, 258–265 (2008).
Breckpot, K. et al. Attenuated expression of A20 markedly increases the efficacy of double-stranded RNA-activated dendritic cells as an anti-cancer vaccine. J. Immunol. 182, 860–870 (2009).
Werner, S.L. et al. Encoding NF-κB temporal control in response to TNF: distinct roles for the negative regulators IκBα and A20. Genes Dev. 22, 2093–2101 (2008).
Hanada, T. et al. Suppressor of cytokine signaling-1 is essential for suppressing dendritic cell activation and systemic autoimmunity. Immunity 19, 437–450 (2003).
Deane, J.A. et al. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity 27, 801–810 (2007).
Adrianto, I. et al. Association of a functional variant downstream of TNFAIP3 with systemic lupus erythematosus. Nat. Genet. 43, 253–258 (2011).
Melis, L. & Elewaut, D. Progress in spondylarthritis. Immunopathogenesis of spondyloarthritis: which cells drive disease? Arthritis Res. Ther. 11, 233 (2009).
Armaka, M. et al. Mesenchymal cell targeting by TNF as a common pathogenic principle in chronic inflammatory joint and intestinal diseases. J. Exp. Med. 205, 331–337 (2008).
Keller, C., Webb, A. & Davis, J. Cytokines in the seronegative spondyloarthropathies and their modification by TNF blockade: a brief report and literature review. Ann. Rheum. Dis. 62, 1128–1132 (2003).
Archer, J.R. Ankylosing spondylitis, IgA, and transforming growth factors. Ann. Rheum. Dis. 54, 544–546 (1995).
Smith, J.A. et al. Gene expression analysis of macrophages derived from ankylosing spondylitis patients reveals interferondysregulation. Arthritis Rheum. 58, 1640–1649 (2008).
Duan, R., Leo, P., Bradbury, L., Brown, M.A. & Thomas, G. Gene expression profiling reveals a downregulation in immune-associated genes in patients with AS. Ann. Rheum. Dis. 69, 1724–1729 (2010).
Acknowledgements
This work was supported by the US National Institutes of Health (A.M.), The Crohn's and Colitis Foundation of America (A.M. and S.O.), The Damon Runyon Cancer Research Foundation (G.E.H.), the Kenneth Rainin Foundation and the UCSF Liver Center and the Wellcome Trust (076113 and 085475 to the Wellcome Trust Case-Control Consortium). A full list of investigators who contributed to this work is available from the Wellcome Trust Case-Control Consortium website.
Author information
Authors and Affiliations
Contributions
G.E.H. designed and did experiments with A20fl/flCd11c-Cre and related mice; E.E.T. and B.A.M. generated A20fl/flCd11c-Cre mice; E.E.T. initiated analyses of A20fl/flCd11c-Cre mice; B.R. generated Cd11c-Cre mice; B.H. and A.D. generated Myd88fl/fl mice; S.O. assisted with colitis experiments; K.E.T. and L.A.C. did genetic analyses of A20 SNPs with data from the Wellcome Trust; C.J.F., E.J.H. and M.C.N. did micro–computed tomography scans and histological analyses of arthritic joints; R.A. and J.B. assisted with breeding, genotyping and radiation chimera experiments; A.M. directed the study; and A.M. and G.E.H., with input from B.A.M., wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 (PDF 1961 kb)
Rights and permissions
About this article
Cite this article
Hammer, G., Turer, E., Taylor, K. et al. Expression of A20 by dendritic cells preserves immune homeostasis and prevents colitis and spondyloarthritis. Nat Immunol 12, 1184–1193 (2011). https://doi.org/10.1038/ni.2135
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2135
This article is cited by
-
Tumor necrosis factor induced protein 3 gene polymorphism and the susceptibility to chronic primary immune thrombocytopenia in Egyptian children: a case-control study
Egyptian Journal of Medical Human Genetics (2021)
-
Targeting ubiquitin signaling for cancer immunotherapy
Signal Transduction and Targeted Therapy (2021)
-
The deubiquitinase OTUB1 augments NF-κB-dependent immune responses in dendritic cells in infection and inflammation by stabilizing UBC13
Cellular & Molecular Immunology (2021)
-
Ankylosing spondylitis: an autoimmune or autoinflammatory disease?
Nature Reviews Rheumatology (2021)
-
Non-catalytic ubiquitin binding by A20 prevents psoriatic arthritis–like disease and inflammation
Nature Immunology (2020)