Abstract
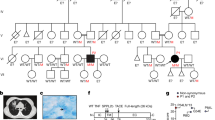
Germline mutations in CYBB, the human gene encoding the gp91phox subunit of the phagocyte NADPH oxidase, impair the respiratory burst of all types of phagocytes and result in X-linked chronic granulomatous disease (CGD). We report here two kindreds in which otherwise healthy male adults developed X-linked recessive Mendelian susceptibility to mycobacterial disease (MSMD) syndromes. These patients had previously unknown mutations in CYBB that resulted in an impaired respiratory burst in monocyte-derived macrophages but not in monocytes or granulocytes. The macrophage-specific functional consequences of the germline mutation resulted from cell-specific impairment in the assembly of the NADPH oxidase. This 'experiment of nature' indicates that CYBB is associated with MSMD and demonstrates that the respiratory burst in human macrophages is a crucial mechanism for protective immunity to tuberculous mycobacteria.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Casanova, J.L. & Abel, L. Genetic dissection of immunity to mycobacteria: the human model. Annu. Rev. Immunol. 20, 581–620 (2002).
Alcais, A., Fieschi, C., Abel, L. & Casanova, J.L. Tuberculosis in children and adults: two distinct genetic diseases. J. Exp. Med. 202, 1617–1621 (2005).
Filipe-Santos, O. et al. Inborn errors of IL-12/23- and IFN-γ-mediated immunity: molecular, cellular, and clinical features. Semin. Immunol. 18, 347–361 (2006).
Bustamante, J. et al. A novel X-linked recessive form of Mendelian susceptibility to mycobaterial disease. J. Med. Genet. 44, e65 (2007).
Filipe-Santos, O. et al. X-linked susceptibility to mycobacteria is caused by mutations in NEMO impairing CD40-dependent IL-12 production. J. Exp. Med. 203, 1745–1759 (2006).
Notarangelo, L.D. et al. Primary immunodeficiencies: 2009 update. J. Allergy Clin. Immunol. 124, 1161–1178 (2009).
Roos, D. et al. Hematologically important mutations: X-linked chronic granulomatous disease (third update). Blood Cells Mol. Dis. 45, 246–265 (2010).
Mouy, R., Fischer, A., Vilmer, E., Seger, R. & Griscelli, C. Incidence, severity, and prevention of infections in chronic granulomatous disease. J. Pediatr. 114, 555–560 (1989).
Segal, B.H., Leto, T.L., Gallin, J.I., Malech, H.L. & Holland, S.M. Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine (Baltimore) 79, 170–200 (2000).
Winkelstein, J.A. et al. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 79, 155–169 (2000).
Bustamante, J. et al. BCG-osis and tuberculosis in a child with chronic granulomatous disease. J. Allergy Clin. Immunol. 120, 32–38 (2007).
Lee, P.P. et al. Susceptibility to mycobacterial infections in children with X-linked chronic granulomatous disease: a review of 17 patients living in a region endemic for tuberculosis. Pediatr. Infect. Dis. J. 27, 224–230 (2008).
van den Berg, J.M. et al. Chronic granulomatous disease: the European experience. PLoS ONE 4, e5234 (2009).
Mohanty, J.G., Jaffe, J.S., Schulman, E.S. & Raible, D.G. A highly sensitive fluorescent micro-assay of H2O2 release from activated human leukocytes using a dihydroxyphenoxazine derivative. J. Immunol. Methods 202, 133–141 (1997).
Nakagawara, A., Nathan, C.F. & Cohn, Z.A. Hydrogen peroxide metabolism in human monocytes during differentiation in vitro. J. Clin. Invest. 68, 1243–1252 (1981).
Martinez, F.O., Gordon, S., Locati, M. & Mantovani, A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J. Immunol. 177, 7303–7311 (2006).
Reichenbach, J. et al. Mycobacterial diseases in primary immunodeficiencies. Curr. Opin. Allergy Clin. Immunol. 1, 503–511 (2001).
Conley, M.E. et al. Primary B cell immunodeficiencies: comparisons and contrasts. Annu. Rev. Immunol. 27, 199–227 (2009).
Condino-Neto, A. & Newburger, P.E. NADPH oxidase activity and cytochrome b558 content of human Epstein-Barr-virus-transformed B lymphocytes correlate with expression of genes encoding components of the oxidase system. Arch. Biochem. Biophys. 360, 158–164 (1998).
Porter, C.D. et al. p22-phox-deficient chronic granulomatous disease: reconstitution by retrovirus-mediated expression and identification of a biosynthetic intermediate of gp91-phox. Blood 84, 2767–2775 (1994).
Maly, F.E. et al. Restitution of superoxide generation in autosomal cytochrome-negative chronic granulomatous disease (A22(0) CGD)-derived B lymphocyte cell lines by transfection with p22phax cDNA. J. Exp. Med. 178, 2047–2053 (1993).
Yu, L. et al. Biosynthesis of flavocytochrome b558. gp91phox is synthesized as a 65-kDa precursor (p65) in the endoplasmic reticulum. J. Biol. Chem. 274, 4364–4369 (1999).
DeLeo, F.R. et al. Processing and maturation of flavocytochrome b558 include incorporation of heme as a prerequisite for heterodimer assembly. J. Biol. Chem. 275, 13986–13993 (2000).
Yu, L., Zhen, L. & Dinauer, M.C. Biosynthesis of the phagocyte NADPH oxidase cytochrome b558. Role of heme incorporation and heterodimer formation in maturation and stability of gp91phox and p22phox subunits. J. Biol. Chem. 272, 27288–27294 (1997).
Nakamura, M., Murakami, M., Koga, T., Tanaka, Y. & Minakami, S. Monoclonal antibody 7D5 raised to cytochrome b558 of human neutrophils: immunocytochemical detection of the antigen in peripheral phagocytes of normal subjects, patients with chronic granulomatous disease, and their carrier mothers. Blood 69, 1404–1408 (1987).
Biberstine-Kinkade, K.J. et al. Heme-ligating histidines in flavocytochrome b(558): identification of specific histidines in gp91phox. J. Biol. Chem. 276, 31105–31112 (2001).
Heyworth, P.G. et al. Hematologically important mutations: X-linked chronic granulomatous disease (second update). Blood Cells Mol. Dis. 27, 16–26 (2001).
Porter, C.D., Kuribayashi, F., Parkar, M.H., Roos, D. & Kinnon, C. Detection of gp91-phox precursor protein in B-cell lines from patients with X-linked chronic granulomatous disease as an indicator for mutations impairing cytochrome b558 biosynthesis. Biochem. J. 315, 571–575 (1996).
Yamauchi, A. et al. Location of the epitope for 7D5, a monoclonal antibody raised against human flavocytochrome b558, to the extracellular peptide portion of primate gp91phox. Microbiol. Immunol. 45, 249–257 (2001).
Burritt, J.B. et al. Phage display epitope mapping of human neutrophil flavocytochrome b558. Identification of two juxtaposed extracellular domains. J. Biol. Chem. 276, 2053–2061 (2001).
Cassatella, M.A. et al. Molecular basis of interferon-γ and lipopolysaccharide enhancement of phagocyte respiratory burst capability. Studies on the gene expression of several NADPH oxidase components. J. Biol. Chem. 265, 20241–20246 (1990).
Ezekowitz, R.A., Dinauer, M.C., Jaffe, H.S., Orkin, S.H. & Newburger, P.E. Partial correction of the phagocyte defect in patients with X-linked chronic granulomatous disease by subcutaneous interferon γ. N. Engl. J. Med. 319, 146–151 (1988).
Ding, C. et al. High-level reconstitution of respiratory burst activity in a human X-linked chronic granulomatous disease (X-CGD) cell line and correction of murine X–CGD bone marrow cells by retroviral-mediated gene transfer of human gp91phox. Blood 88, 1834–1840 (1996).
Ezekowitz, R.A. et al. Restoration of phagocyte function by interferon-γ in X-linked chronic granulomatous disease occurs at the level of a progenitor cell. Blood 76, 2443–2448 (1990).
Zhen, L. et al. Gene targeting of X chromosome-linked chronic granulomatous disease locus in a human myeloid leukemia cell line and rescue by expression of recombinant gp91phox. Proc. Natl. Acad. Sci. USA 90, 9832–9836 (1993).
Casbon, A.J., Allen, L.A., Dunn, K.W. & Dinauer, M.C. Macrophage NADPH oxidase flavocytochrome B localizes to the plasma membrane and Rab11-positive recycling endosomes. J. Immunol. 182, 2325–2339 (2009).
Zhu, Y. et al. Deletion mutagenesis of p22phox subunit of flavocytochrome b558: identification of regions critical for gp91phox maturation and NADPH oxidase activity. J. Biol. Chem. 281, 30336–30346 (2006).
Biberstine-Kinkade, K.J. et al. Mutagenesis of p22phox histidine 94. A histidine in this position is not required for flavocytochrome b558 function. J. Biol. Chem. 277, 30368–30374 (2002).
Perkins, S.L., Link, D.C., Kling, S., Ley, T.J. & Teitelbaum, S.L. 1,25-Dihydroxyvitamin D3 induces monocytic differentiation of the PLB-985 leukemic line and promotes c-fgr mRNA expression. J. Leukoc. Biol. 50, 427–433 (1991).
Jitkaew, S., Witasp, E., Zhang, S., Kagan, V.E. & Fadeel, B. Induction of caspase- and reactive oxygen species-independent phosphatidylserine externalization in primary human neutrophils: role in macrophage recognition and engulfment. J. Leukoc. Biol. 85, 427–437 (2009).
Savina, A. et al. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell 126, 205–218 (2006).
Adams, L.B., Dinauer, M.C., Morgenstern, D.E. & Krahenbuhl, J.L. Comparison of the roles of reactive oxygen and nitrogen intermediates in the host response to Mycobacterium tuberculosis using transgenic mice. Tuber. Lung Dis. 78, 237–246 (1997).
Segal, B.H. et al. The p47(phox−/−) mouse model of chronic granulomatous disease has normal granuloma formation and cytokine responses to Mycobacterium avium and Schistosoma mansoni eggs. Infect. Immun. 67, 1659–1665 (1999).
Nathan, C. & Shiloh, M.U. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. USA 97, 8841–8848 (2000).
MacMicking, J., Xie, Q.W. & Nathan, C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 15, 323–350 (1997).
Casanova, J.L. & Abel, L. Primary immunodeficiencies: a field in its infancy. Science 317, 617–619 (2007).
Alcais, A., Abel, L. & Casanova, J.L. Human genetics of infectious diseases: between proof of principle and paradigm. J. Clin. Invest. 119, 2506–2514 (2009).
Gordon, S. & Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964 (2005).
Segal, A.W. How neutrophils kill microbes. Annu. Rev. Immunol. 23, 197–223 (2005).
Price, M.O. et al. Creation of a genetic system for analysis of the phagocyte respiratory burst: high-level reconstitution of the NADPH oxidase in a nonhematopoietic system. Blood 99, 2653–2661 (2002).
Acknowledgements
We thank the family members for participating in this study; J. Curnutte (3-V Biosciences) for EBV-B cells from CGD controls; D. Roos (University of Amsterdam) for mAb 449; F. Morel (Grenoble University) for mAb 7A2; M. Quinn (Montana State University) for mAb 54.1; all members of the two branches of the laboratory of Human Genetics of Infectious Diseases for discussions; and T. Leclerc, Y. Rose, M. de Suremain, M. Kezadi, and N. Stull for technical assistance. Supported by Institut National de la Santé et de la Recherche Médicale (J.B.), the European Union (NEOTIM EEA05095KKA to J.B., and HOMITB HEALTH-F3-2008-200732), the March of Dimes (RO5050KK to J.B.), Fundação de Amparo a Pesquisa do Estado de São Paulo (A.C.-N.), Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (A.C.-N.), Fondation BNP-Paribas, Fondation Schlumberger, Institut Universitaire de France, Agence Nationale de Recherche, The Rockefeller University Center for Clinical and Translational Science (5UL1RR024143-03), The Rockefeller University, the National Institutes of Health (AI 079788 and DK54369 to P.E.N., and HL045635 to M.C.D.), the Riley Children's Foundation (M.C.D.) and the Howard Hughes Medical Institute (J.-L.C.).
Author information
Authors and Affiliations
Contributions
J.B., L.A. and J.-L.C. designed the study and contributed intellectually to the experimental process; J.B. did most of the experiments under the supervision of J.-L.C.; A.A.A., C.C.M., E.V. and M.C.D. did the experiments with retroviral transduction of gp91phox into EBV-B, CHO and PLB-985 cells; G.V. made the nonretroviral CYBB vectors, infected macrophages with BCG and made intellectual contributions to various experiments; C. Picard contributed to the recruitment of patients and initiated the clinical investigation; L.B.G., C. Prando, L.J. and M.H. analyzed many controls; A.C. did quantitative RT-PCR; L.d.B. did bioinformatics analysis; J.-F.E. did histological analysis of lymph nodes; B.W. did immunoperoxidase staining; A.V.G., C.B. and A.B. provided data for linkage analysis; A.P., J.F. and S.B.-D. provided experimental advice about cell culture; J.-M.B., S.B., O.L. and C.F. contributed to the recruitment and follow-up of the patients and CGD controls; S.A.-M., P.N., A.C.-N. and M.C.D. provided CGD controls and intellectual guidance for the development of various assays; J.B. and J.-L.C. wrote the paper; and all authors commented on and discussed the paper. B.W., P.E.N., A.C.-N. and M.C.D. contributed equally to this work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 1573 kb)
Rights and permissions
About this article
Cite this article
Bustamante, J., Arias, A., Vogt, G. et al. Germline CYBB mutations that selectively affect macrophages in kindreds with X-linked predisposition to tuberculous mycobacterial disease. Nat Immunol 12, 213–221 (2011). https://doi.org/10.1038/ni.1992
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1992
This article is cited by
-
Mendelian susceptibility to mycobacterial disease: an overview
Egyptian Journal of Medical Human Genetics (2023)
-
Distinct Lymphocyte Immunophenotyping and Quantitative Anti-Interferon Gamma Autoantibodies in Taiwanese HIV-Negative Patients with Non-Tuberculous Mycobacterial Infections
Journal of Clinical Immunology (2023)
-
Tuberculosis and Bacillus Calmette-Guérin Disease in Patients with Chronic Granulomatous Disease: an Experience from a Tertiary Care Center in North India
Journal of Clinical Immunology (2023)
-
Chronic Granulomatous Disease-Like Presentation of a Child with Autosomal Recessive PKCδ Deficiency
Journal of Clinical Immunology (2022)
-
Disseminated Tuberculosis in a Patient with Autosomal Recessive p47phox Chronic Granulomatous Disease
Journal of Clinical Immunology (2021)