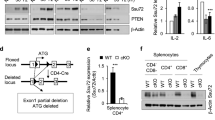
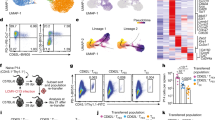
Abstract
Naive CD4+ T cells differentiate into diverse effector and regulatory lineages to orchestrate immunity and tolerance. Here we found that the differentiation of proinflammatory T helper type 1 (TH1) cells and anti-inflammatory Foxp3+ regulatory T cells (Treg cells) was reciprocally regulated by S1P1, a receptor for the bioactive lipid sphingosine 1-phosphate (S1P). S1P1 inhibited the generation of extrathymic and natural Treg cells while driving TH1 development in a reciprocal manner and disrupted immune homeostasis. S1P1 signaled through the kinase mTOR and antagonized the function of transforming growth factor-β mainly by attenuating sustained activity of the signal transducer Smad3. S1P1 function was dependent on endogenous sphingosine kinase activity. Notably, two seemingly unrelated immunosuppressants, FTY720 and rapamycin, targeted the same S1P1 and mTOR pathway to regulate the dichotomy between TH1 cells and Treg cells. Our studies establish an S1P1-mTOR axis that controls T cell lineage specification.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Zhou, L., Chong, M.M. & Littman, D.R. Plasticity of CD4+ T cell lineage differentiation. Immunity 30, 646–655 (2009).
Curotto de Lafaille, M.A. & Lafaille, J.J. Natural and adaptive Foxp3+ regulatory T cells: more of the same or a division of labor? Immunity 30, 626–635 (2009).
Josefowicz, S.Z. & Rudensky, A. Control of regulatory T cell lineage commitment and maintenance. Immunity 30, 616–625 (2009).
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006).
Mucida, D. et al. Reciprocal Th-17 and regulatory T cell differentiation mediated by retinoic acid. Science 317, 256–260 (2007).
Sun, C.M. et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 204, 1775–1785 (2007).
Coombes, J.L. et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J. Exp. Med. 204, 1757–1764 (2007).
Veldhoen, M. et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453, 106–109 (2008).
Quintana, F.J. et al. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature 453, 65–71 (2008).
Rivera, J., Proia, R.L. & Olivera, A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat. Rev. Immunol. 8, 753–763 (2008).
Kappos, L. et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N. Engl. J. Med. 362, 387–401 (2010).
Matloubian, M. et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427, 355–360 (2004).
Chi, H. & Flavell, R.A. Cutting edge: regulation of T cell trafficking and primary immune responses by sphingosine 1-phosphate receptor 1. J. Immunol. 174, 2485–2488 (2005).
Liu, G. et al. The receptor S1P1 overrides regulatory T cell–mediated immune suppression through Akt-mTOR. Nat. Immunol. 10, 769–777 (2009).
Chen, W. et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 198, 1875–1886 (2003).
Selvaraj, R.K. & Geiger, T.L. A kinetic and dynamic analysis of Foxp3 induced in T cells by TGF-β. J. Immunol. 178, 7667–7677 (2007).
Li, M.O. & Flavell, R.A. TGF-β: a master of all T cell trades. Cell 134, 392–404 (2008).
Izcue, A., Coombes, J.L. & Powrie, F. Regulatory lymphocytes and intestinal inflammation. Annu. Rev. Immunol. 27, 313–338 (2009).
Haribhai, D. et al. A central role for induced regulatory T cells in tolerance induction in experimental colitis. J. Immunol. 182, 3461–3468 (2009).
Wei, J. et al. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc. Natl. Acad. Sci. USA 104, 18169–18174 (2007).
Wang, Z. et al. Role of IFN-γ in induction of Foxp3 and conversion of CD4+CD25−s T cells to CD4+ Tregs. J. Clin. Invest. 116, 2434–2441 (2006).
Yang, X.O. et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity 29, 44–56 (2008).
Gorelik, L. & Flavell, R.A. Abrogation of TGFβ signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity 12, 171–181 (2000).
Fahlen, L. et al. T cells that cannot respond to TGF-β escape control by CD4+CD25+ regulatory T cells. J. Exp. Med. 201, 737–746 (2005).
Li, M.O., Sanjabi, S. & Flavell, R.A. Transforming growth factor-β controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity 25, 455–471 (2006).
Marie, J.C., Liggitt, D. & Rudensky, A.Y. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-β receptor. Immunity 25, 441–454 (2006).
Tone, Y. et al. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat. Immunol. 9, 194–202 (2008).
Haxhinasto, S., Mathis, D. & Benoist, C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J. Exp. Med. 205, 565–574 (2008).
Sauer, S. et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc. Natl. Acad. Sci. USA 105, 7797–7802 (2008).
Delgoffe, G.M. et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 30, 832–844 (2009).
Thomson, A.W., Turnquist, H.R. & Raimondi, G. Immunoregulatory functions of mTOR inhibition. Nat. Rev. Immunol. 9, 324–337 (2009).
Sehrawat, S. & Rouse, B.T. Anti-inflammatory effects of FTY720 against viral-induced immunopathology: role of drug-induced conversion of T cells to become Foxp3+ regulators. J. Immunol. 180, 7636–7647 (2008).
Ouyang, W., Beckett, O., Ma, Q. & Li, M.O. Transforming growth factor-β signaling curbs thymic negative selection promoting regulatory T cell development. Immunity 32, 642–653 (2010).
Takabe, K., Paugh, S.W., Milstien, S. & Spiegel, S. 'Inside-out' signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol. Rev. 60, 181–195 (2008).
Zhou, L. et al. TGF-β-induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORγt function. Nature 453, 236–240 (2008).
Zhang, F., Meng, G. & Strober, W. Interactions among the transcription factors Runx1, RORγt and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nat. Immunol. 9, 1297–1306 (2008).
Kitano, M. et al. Sphingosine 1-phosphate/sphingosine 1-phosphate receptor 1 signaling in rheumatoid synovium: regulation of synovial proliferation and inflammatory gene expression. Arthritis Rheum. 54, 742–753 (2006).
Zachariah, M.A. & Cyster, J.G. Neural crest-derived pericytes promote egress of mature thymocytes at the corticomedullary junction. Science 328, 1129–1135 (2010).
Liu, Y. et al. A critical function for TGF-β signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat. Immunol. 9, 632–640 (2008).
Martinez, G.J. et al. Smad3 differentially regulates the induction of regulatory and inflammatory T cell differentiation. J. Biol. Chem. 284, 35283–35286 (2009).
Wang, W., Huang, M.C. & Goetzl, E.J. Type 1 sphingosine 1-phosphate G protein-coupled receptor (S1P1) mediation of enhanced IL-4 generation by CD4 T cells from S1P1 transgenic mice. J. Immunol. 178, 4885–4890 (2007).
Huang, M.C., Watson, S.R., Liao, J.J. & Goetzl, E.J. Th17 augmentation in OTII TCR plus T cell-selective type 1 sphingosine 1-phosphate receptor double transgenic mice. J. Immunol. 178, 6806–6813 (2007).
Lee, K. et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity 32, 743–753 (2010).
Merkenschlager, M. & von Boehmer, H. PI3 kinase signalling blocks Foxp3 expression by sequestering Foxo factors. J. Exp. Med. 207, 1347–1350 (2010).
Song, K., Wang, H., Krebs, T.L. & Danielpour, D. Novel roles of Akt and mTOR in suppressing TGF-β/ALK5-mediated Smad3 activation. EMBO J. 25, 58–69 (2006).
Seoane, J., Le, H.V., Shen, L., Anderson, S.A. & Massague, J. Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell 117, 211–223 (2004).
Pappu, R. et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science 316, 295–298 (2007).
Liu, X. et al. Transforming growth factor β-induced phosphorylation of Smad3 is required for growth inhibition and transcriptional induction in epithelial cells. Proc. Natl. Acad. Sci. USA 94, 10669–10674 (1997).
Yang, K. et al. Hsp90 regulates activation of interferon regulatory factor 3 and TBK-1 stabilization in Sendai virus-infected cells. Mol. Biol. Cell 17, 1461–1471 (2006).
Acknowledgements
We thank R. Proia (US National Institutes of Health) for S1pr1flox/flox mice; A. Rudensky (Memorial Sloan-Kettering Institute) for Foxp3gfp knock-in mice; T. Ludwig (Columbia University) for Rosa26-Cre-ERT2 mice; D. Littman (New York University) for Foxp3-expressing retroviral constructs; D. Green (St. Jude Children's Research Hospital) for Bcl-2-transgenic mice and discussions; and R. Cross, G. Lennon and S. Morgan for cell sorting. Supported by US National Institutes of Health (K01 AR053573 and administrative supplement, R01 NS064599, and Cancer Center Support Grant CA021765), the Arthritis Foundation, the Lupus Research Institute and the American Lebanese Syrian Associated Charities (H.C.).
Author information
Authors and Affiliations
Contributions
G.L. designed and did in vivo and cellular experiments and contributed to the writing of the manuscript; K.Y. designed and did biochemical analyses and cellular and molecular experiments; S.B. did in vivo and cellular experiments and gene-expression analysis; S.S. contributed to cell isolation and gene expression analysis and managed the mouse colony; and H.C. designed experiments, wrote the manuscript and provided overall direction.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–13 (PDF 992 kb)
Rights and permissions
About this article
Cite this article
Liu, G., Yang, K., Burns, S. et al. The S1P1-mTOR axis directs the reciprocal differentiation of TH1 and Treg cells. Nat Immunol 11, 1047–1056 (2010). https://doi.org/10.1038/ni.1939
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1939
This article is cited by
-
How autoreactive thymocytes differentiate into regulatory versus effector CD4+ T cells after avoiding clonal deletion
Nature Immunology (2023)
-
The role of sphingosine-1-phosphate in autophagy and related disorders
Cell Death Discovery (2023)
-
S1P/S1PR1 signaling differentially regulates the allogeneic response of CD4 and CD8 T cells by modulating mitochondrial fission
Cellular & Molecular Immunology (2022)
-
Sphingosine 1-phosphate modulation and immune cell trafficking in inflammatory bowel disease
Nature Reviews Gastroenterology & Hepatology (2022)
-
The effect of fingolimod on regulatory T cells in a mouse model of brain ischaemia
Journal of Neuroinflammation (2021)