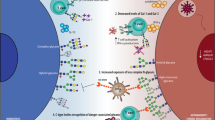
Abstract
Cooperation between different innate signaling pathways induced by pattern-recognition receptors (PRRs) on dendritic cells (DCs) is crucial for tailoring adaptive immunity to pathogens. Here we show that carbohydrate-specific signaling through the C-type lectin DC-SIGN tailored cytokine production in response to distinct pathogens. DC-SIGN was constitutively associated with a signalosome complex consisting of the scaffold proteins LSP1, KSR1 and CNK and the kinase Raf-1. Mannose-expressing Mycobacterium tuberculosis and human immunodeficiency virus type 1 (HIV-1) induced the recruitment of effector proteins to the DC-SIGN signalosome to activate Raf-1, whereas fucose-expressing pathogens such as Helicobacter pylori actively dissociated the KSR1–CNK–Raf-1 complex from the DC-SIGN signalosome. This dynamic regulation of the signalosome by mannose- and fucose-expressing pathogens led to the enhancement or suppression of proinflammatory responses, respectively. Our study reveals another level of plasticity in tailoring adaptive immunity to pathogens.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ueno, H. et al. Dendritic cell subsets in health and disease. Immunol. Rev. 219, 118–142 (2007).
Medzhitov, R. Recognition of microorganisms and activation of the immune response. Nature 449, 819–826 (2007).
Wilson, C.B., Rowell, E. & Sekimata, M. Epigenetic control of T-helper-cell differentiation. Nat. Rev. Immunol. 9, 91–105 (2009).
Kanneganti, T.D., Lamkanfi, M. & Núñez, G. Intracellular NOD-like receptors in host defense and disease. Immunity 27, 549–559 (2007).
Meylan, E. & Tschopp, J. Toll-Like Receptors and RNA helicases: two parallel ways to trigger antiviral responses. Mol. Cell 22, 561–569 (2006).
O'Neill, L.A.J. When signaling pathways collide: positive and negative regulation of Toll-like receptor signal transduction. Immunity 29, 12–20 (2008).
Robinson, M.J., Sancho, D., Slack, E.C., LeibundGut-Landmann, S. & Sousa, C.R.E. Myeloid C-type lectins in innate immunity. Nat. Immunol. 7, 1258–1265 (2006).
Sansonetti, P.J. The innate signaling of dangers and the dangers of innate signaling. Nat. Immunol. 7, 1237–1242 (2006).
Kawai, T. & Akira, S. TLR signaling. Cell Death Differ. 13, 816–825 (2006).
O'Neill, L.A.J. & Bowie, A.G. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 7, 353–364 (2007).
Ouaaz, F., Arron, J., Zheng, Y., Choi, Y. & Beg, A.A. Dendritic cell development and survival require distinct NF-κB subunits. Immunity 16, 257–270 (2002).
Geijtenbeek, T.B.H. et al. Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 197, 7–17 (2003).
Underhill, D.M. Collaboration between the innate immune receptors dectin-1, TLRs, and Nods. Immunol. Rev. 219, 75–87 (2007).
Geijtenbeek, T.B.H. & Gringhuis, S.I. Signalling through C-type lectin receptors: shaping immune responses. Nat. Rev. Immunol. 9, 465–479 (2009).
Appelmelk, B.J. et al. Carbohydrate profiling identifies new pathogens that interact with dendritic cell-specific ICAM-3-grabbing nonintegrin on dendritic cells. J. Immunol. 170, 1635–1639 (2003).
van Kooyk, Y. & Geijtenbeek, T.B.H. DC-SIGN: escape mechanism for pathogens. Nat. Rev. Immunol. 3, 697–709 (2003).
Feinberg, H., Mitchell, D.A., Drickamer, K. & Weis, W.I. Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science 294, 2163–2166 (2001).
Bergman, M.P. et al. Helicobacter pylori modulates the T helper cell 1/T helper cell 2 balance through phase-variable interaction between lipopolysaccharide and DC-SIGN. J. Exp. Med. 200, 979–990 (2004).
Smits, H.H. et al. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J. Allergy Clin. Immunol. 115, 1260–1267 (2005).
Steeghs, L. et al. Neisseria meningitidis expressing lgtB lipopolysaccharide targets DC-SIGN and modulates dendritic cell function. Cell. Microbiol. 8, 316–325 (2006).
van Liempt, E. et al. Schistosoma mansoni soluble egg antigens are internalized by human dendritic cells through multiple C-type lectins and suppress TLR-induced dendritic cell activation. Mol. Immunol. 44, 2605–2615 (2007).
Gringhuis, S.I. et al. C-type lectin DC-SIGN modulates toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-κB. Immunity 26, 605–616 (2007).
Wellbrock, C., Karasarides, M. & Marais, R. The Raf proteins take centre stage. Nat. Rev. Mol. Cell Biol. 5, 875–885 (2004).
Geijtenbeek, T.B.H. et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100, 587–597 (2000).
Hong, P.W.P. et al. Human immunodeficiency virus envelope (gp120) binding to DC-SIGN and primary dendritic cells is carbohydrate dependent but does not involve 2G12 or cyanovirin binding sites: implications for structural analyses of gp120-DC-SIGN binding. J. Virol. 76, 12855–12865 (2002).
Geijtenbeek, T.B. et al. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 100, 575–585 (2000).
van Gisbergen, K.P.J.M., Sanchez-Hernandez, M., Geijtenbeek, T.B.H. & van Kooyk, Y. Neutrophils mediate immune modulation of dendritic cells through glycosylation-dependent interactions between Mac-1 and DC-SIGN. J. Exp. Med. 201, 1281–1292 (2005).
Smith, A.L. et al. Leukocyte-specific protein 1 interacts with DC-SIGN and mediates transport of HIV to the proteasome in dendritic cells. J. Exp. Med. 204, 421–430 (2007).
Harrison, R.E., Sikorski, B.A. & Jongstra, J. Leukocyte-specific protein 1 targets the ERK/MAP kinase scaffold protein KSR and MEK1 and ERK2 to the actin cytoskeleton. J. Cell Sci. 117, 2151–2157 (2004).
Douziech, M., Sahmi, M., Laberge, G. & Therrien, M.A. KSR/CNK complex mediated by HYP, a novel SAM domain-containing protein, regulates RAS-dependent RAF activation in Drosophila. Genes Dev. 20, 807–819 (2006).
Hodges, A. et al. Activation of the lectin DC-SIGN induces an immature dendritic cell phenotype triggering Rho-GTPase activity required for HIV-1 replication. Nat. Immunol. 8, 569–577 (2007).
Dhillon, A.S., von, K.A., Grindlay, J. & Kolch, W. Phosphatase and feedback regulation of Raf-1 signaling. Cell Cycle 6, 3–7 (2007).
Yadav, M. & Schorey, J.S. The β-glucan receptor dectin-1 functions together with TLR2 to mediate macrophage activation by mycobacteria. Blood 108, 3168–3175 (2006).
Appelmelk, B.J. et al. The mannose cap of mycobacterial lipoarabinomannan does not dominate the Mycobacterium-host interaction. Cell. Microbiol. 10, 930–944 (2008).
Shaw, A.S. & Filbert, E.L. Scaffold proteins and immune-cell signalling. Nat. Rev. Immunol. 9, 47–56 (2009).
Dhanasekaran, D.N., Kashef, K., Lee, C.M., Xu, H. & Reddy, E.P. Scaffold proteins of MAP-kinase modules. Oncogene 26, 3185–3202 (2007).
Taya, S. et al. Direct interaction of insulin-like growth factor-1 receptor with leukemia-associated RhoGEF. J. Cell Biol. 155, 809–820 (2001).
Jaffe, A.B., Aspenstrom, P. & Hall, A. Human CNK1 acts as a scaffold protein, linking Rho and Ras signal transduction pathways. Mol. Cell. Biol. 24, 1736–1746 (2004).
Ziogas, A., Moelling, K. & Radziwill, G. CNK1 is a scaffold protein that regulates Src-mediated Raf-1 activation. J. Biol. Chem. 280, 24205–24211 (2005).
Kolch, W. Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat. Rev. Mol. Cell Biol. 6, 827–837 (2005).
Matheny, S.A. et al. Ras regulates assembly of mitogenic signalling complexes through the effector protein IMP. Nature 427, 256–260 (2004).
Guo, Y. et al. Structural basis for distinct ligand-binding and targeting properties of the receptors DC-SIGN and DC-SIGNR. Nat. Struct. Mol. Biol. 11, 591–598 (2004).
Madura Larsen, J. et al. BCG stimulated dendritic cells induce an interleukin-10 producing T-cell population with no T helper 1 or T helper 2 bias in vitro. Immunology 121, 276–282 (2007).
van Die, I. et al. The dendritic cell-specific C-type lectin DC-SIGN is a receptor for Schistosoma mansoni egg antigens and recognizes the glycan antigen Lewis x. Glycobiology 13, 471–478 (2003).
Uematsu, S. & Akira, S. Toll-Like receptors (TLRs) and their ligands. Handb. Exp. Pharmacol. 183, 1–20 (2008).
Hanke, J.H. et al. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J. Biol. Chem. 271, 695–701 (1996).
Gringhuis, S.I. et al. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-κB activation through Raf-1 and Syk. Nat. Immunol. 10, 203–213 (2009).
Remans, P.H.J. et al. Rap1 signaling is required for suppression of Ras-generated reactive oxygen species and protection against oxidative stress in T lymphocytes. J. Immunol. 173, 920–931 (2004).
Acknowledgements
ManLAM and M. tuberculosis were gifts from J. Belisle (Colorado State University; as part of the National Institute of Allergy and Infectious Diseases, US National Institutes of Health contract HHSN266200400091C, “Tuberculosis Vaccine Testing and Research Materials”); H. pylori strains were gifts from B. Appelmelk (Vrije University Medical Center); and ICAM-3–Fc was a gift from D. Simmons (University of Oxford). Supported by the Dutch Scientific Research Program (NWO 912-04-025 to J.d.D.), the AIDS Foundation (2007036 to M.d.v.V.) and the Dutch Asthma Foundation (3.2.03.39 to S.I.G.).
Author information
Authors and Affiliations
Contributions
S.I.G. designed, did and interpreted most experiments and prepared the manuscript; J.d.D. and M.L. participated in RNAi, flow cytometry and immunoblot experiments; M.v.d.V. assisted with flow cytometry and Ras-precipitation experiments; and T.B.H.G. participated in flow cytometry and enzyme-linked immunosorbent assays (ELISAs) and supervised all aspects of this study.
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Table 1 (PDF 750 kb)
Rights and permissions
About this article
Cite this article
Gringhuis, S., den Dunnen, J., Litjens, M. et al. Carbohydrate-specific signaling through the DC-SIGN signalosome tailors immunity to Mycobacterium tuberculosis, HIV-1 and Helicobacter pylori. Nat Immunol 10, 1081–1088 (2009). https://doi.org/10.1038/ni.1778
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1778
This article is cited by
-
SARS CoV-2 spike protein variants exploit DC-SIGN/DC-SIGNR receptor for evolution and severity: an in-silico insight
VirusDisease (2023)
-
Characterization of a CD209-Like Lectin in Black Rockfish (Sebastes schlegelii): Structure Features and Binding Activities as a Pathogen Recognition Receptor
Journal of Ocean University of China (2023)
-
Impact of disease-modifying therapy on dendritic cells and exploring their immunotherapeutic potential in multiple sclerosis
Journal of Neuroinflammation (2022)
-
Fungal sensing by dectin-1 directs the non-pathogenic polarization of TH17 cells through balanced type I IFN responses in human DCs
Nature Immunology (2022)
-
Human anogenital monocyte-derived dendritic cells and langerin+cDC2 are major HIV target cells
Nature Communications (2021)