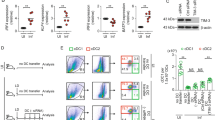
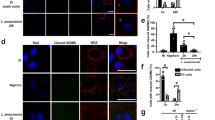
Abstract
CD40, a costimulatory molecule expressed on macrophages, induces expression of interleukin 12 (IL-12) in uninfected macrophages and IL-10 in macrophages infected with Leishmania major. IL-12 suppresses, whereas IL-10 enhances, L. major infection. The mechanisms that regulate this difference in CD40-induced cytokine production remain unclear, but it is known that L. major depletes cholesterol. Here we show that cholesterol influenced the assembly of distinct CD40 signalosomes. Depletion of membrane cholesterol inhibited the assembly of an IL-12-inducing CD40 signalosome containing the adaptors TRAF2, TRAF3 and TRAF5 and the kinase Lyn and promoted the assembly of an IL-10-inducing CD40 signalosome containing the adaptor TRAF6 and the kinase Syk. Thus, cholesterol depletion might represent an immune-evasion strategy used by L. major.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
18 May 2009
NOTE: In the version of this article initially published, the GenBank accession number for the Indian human immunodeficiency virus 2 isolate is incorrect. The correct accession number is DQ307022. The error has been corrected in the HTML and PDF versions of the article.
References
Li, L., Elliott, J.F. & Mosmann, T.R. IL-10 inhibits cytokine production, vascular leakage, and swelling during T helper 1 cell-induced delayed-type hypersensitivity. J. Immunol. 153, 3967–3978 (1994).
Snapper, C.M. & Paul, W.E. Interferon-γ and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science 236, 944–947 (1987).
Mond, J.J., Carman, J., Sarma, C., Ohara, J. & Finkelman, F.D. Interferon-γ suppresses B cell stimulation factor (BSF-1) induction of class II MHC determinants on B cells. J. Immunol. 137, 3534–3537 (1986).
Krummel, M.F. & Allison, J.P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 182, 459–465 (1995).
Walunas, T.L., Bakker, C.Y. & Bluestone, J.A. CTLA-4 ligation blocks CD28-dependent T cell activation. J. Exp. Med. 183, 2541–2550 (1996).
Grammer, A.C. & Lipsky, P.E. CD40-mediated regulation of immune responses by TRAF-dependent and TRAF-independent signaling mechanisms. Adv. Immunol. 76, 61–178 (2000).
Bishop, G.A., Moore, C.R., Xie, P., Stunz, L.L. & Kraus, Z.J. TRAF proteins in CD40 signaling. Adv. Exp. Med. Biol. 597, 131–151 (2007).
Bishop, G.A., Hostager, B.S. & Brown, K.D. Mechanisms of TNF receptor-associated factor (TRAF) regulation in B lymphocytes. J. Leukoc. Biol. 72, 19–23 (2002).
Kamanaka, M. et al. Protective role of CD40 in Leishmania major infection at two distinct phases of cell-mediated immunity. Immunity 4, 275–281 (1996).
Soong, L. et al. Disruption of CD40–CD40 ligand interactions results in an enhanced susceptibility to Leishmania amazonensis infection. Immunity 4, 263–273 (1996).
Campbell, K.A. et al. CD40 ligand is required for protective cell-mediated immunity to Leishmania major. Immunity 4, 283–289 (1996).
Mathur, R.K., Awasthi, A., Wadhone, P., Ramanamurthy, B. & Saha, B. Reciprocal CD40 signals through p38MAPK and Erk-1/2 induce counteracting immune responses. Nat. Med. 10, 540–544 (2004); erratum in Nat. Med. 10, 755 (2004).
Chakraborty, D. et al. Leishmania donovani affects antigen presentation of macrophage by disrupting lipid rafts. J. Immunol. 175, 3214–3224 (2005).
Vidalain, P.O. et al. CD40 signaling in human dendritic cells is initiated within membrane rafts. EMBO J. 19, 3304–3313 (2000).
Hancock, J.F. & Patron, R.G. Ras plasma membrane signaling platforms. Biochem. J. 389, 1–11 (2005).
Nicolau, D.V. Jr., Burrage, K., Parton, R.G. & Hancock, J.F. Identifying optimal lipid raft characteristics required to promote nanoscale protein-protein interactions on the plasma membrane. Mol. Cell. Biol. 26, 313–323 (2006).
Hancock, J.F. Lipid rafts: contentious only from simplistic standpoints. Nat. Rev. Mol. Cell Biol. 7, 456–462 (2006).
Pontier, S.M. et al. Cholesterol-dependent separation of the β2-adrenergic receptor from its partners determines signaling efficacy: insight into nanoscale organization of signal transduction. J. Biol. Chem. 283, 24659–24672 (2008).
Frank, C. et al. Cholesterol depletion inhibits synaptic transmission and synaptic plasticity in rat hippocampus. Exp. Neurol. 212, 407–414 (2008).
Pucadyil, T.J. & Chattopadhyay, A. Cholesterol depletion induces dynamic confinement of the G-protein coupled serotonin (1A) receptor in the plasma membrane of living cells. Biochim. Biophys. Acta 1768, 655–668 (2007).
Xia, M. et al. Anthocyanin prevents CD40-activated proinflammatory signaling in endothelial cells by regulating cholesterol distribution. Arterioscler. Thromb. Vasc. Biol. 27, 519–524 (2007).
McConnell, H.M. & Radhakrishnan, A. Condensed complexes of cholesterol and phospholipids. Biochim. Biophys. Acta 1610, 159–173 (2003).
McConnell, H.M. & Vrljic, M. Liquid–liquid immiscibility in membranes. Annu. Rev. Biophys. Biomol. Struct. 32, 469–492 (2003).
Huang, J. & Feigenson, G.W. A microscopic interaction model of maximum solubility of cholesterol in lipid bilayers. Biophys. J. 76, 2142–2157 (1999).
Pandit, S.A., Jakobsson, E. & Scott, H.L. Simulation of the early stages of nano-domain formation in mixed bilayers of sphingomyelin, cholesterol, and dioleylphosphatidylcholine. Biophys. J. 87, 3312–3322 (2004).
Ipsen, J.H. et al. Phase equilibria in the phosphatidylcholine–cholesterol system. Biochim. Biophys. Acta 905, 162–172 (1987).
Pullen, S.S. et al. High-affinity interactions of tumor necrosis factor receptor-associated factors (TRAFs) and CD40 require TRAF trimerization and CD40 multimerization. Biochemistry 38, 10168–10177 (1999).
Awasthi, A. et al. CD40 signaling is impaired in L. major-infected macrophages and is rescued by a p38MAPK activator establishing a host-protective memory T cell response. J. Exp. Med. 197, 1037–1043 (2003).
Pullen, S.S. et al. CD40-tumor necrosis factor receptor-associated factor (TRAF) interactions: regulation of CD40 signaling through multiple TRAF binding sites and TRAF hetero-oligomerization. Biochemistry 37, 11836–11845 (1998).
Sefton, B.M. & Taddie, J.A. Role of tyrosine kinases in lymphocyte activation. Curr. Opin. Immunol. 6, 372–379 (1994).
Waiczies, S. et al. Atorvastatin induces T cell anergy via phosphorylation of ERK1. J. Immunol. 174, 5630–5635 (2005).
Gegg, M.E. et al. Suppression of autoimmune retinal disease by lovastatin does not require Th2 cytokine induction. J. Immunol. 174, 2327–2335 (2005).
Murugaiyan, G., Agrawal, R., Mishra, G.C., Mitra, D. & Saha, B. Functional dichotomy in CD40 reciprocally regulates effector T cell functions. J. Immunol. 177, 6642–6649 (2006).
Murugaiyan, G., Agrawal, R., Mishra, G.C., Mitra, D. & Saha, B. Differential CD40/CD40L expression results in counteracting anti-tumor immune responses. J. Immunol. 178, 2047–2055 (2007).
Toubi, E. & Shoenfeld, Y. The role of CD40–CD154 interactions in autoimmunity and the benefit of disrupting this pathway. Autoimmunity 37, 457–464 (2004).
Quezada, S.A., Jarvinen, L.Z., Lind, E.F. & Noelle, R.J. CD40/CD154 interactions at the interface of tolerance and immunity. Annu. Rev. Immunol. 22, 307–328 (2004).
Yamada, A. & Sayegh, M.H. The CD154–CD40 costimulatory pathway in transplantation. Transplantation 73, S36–S39 (2002).
Lichtenberg, D., Goni, F.M. & Heerklotz, H. Detergent-resistant membranes should not be identified with membrane rafts. Trends Biochem. Sci. 30, 430–436 (2005).
Veiga, M.P. et al. Interaction of cholesterol and sphingomyelin in mixed membranes containing phosphatidylcholine, studied by spin-label ESR and IR spectroscopies. A possible stabilization of gel-phase sphingolipid domains by cholesterol. Biochemistry 40, 2614–2622 (2001).
Scheiffele, P. et al. Interaction of influenza virus haemagglutinin with sphingolipid–cholesterol membrane domains via its transmembrane domain. EMBO J. 16, 5501–5508 (1997).
Bock, J. & Gulbins, E. The transmembranous domain of CD40 determines CD40 partitioning into lipid rafts. FEBS Lett. 534, 169–174 (2003).
Guigas, G. & Weiss, M. Influence of hydrophobic mismatching on membrane protein diffusion. Biophys. J. 95, L25–L27 (2008).
Groux, H. et al. A transgenic model to analyze the immunoregulatory role of IL-10 secreted by antigen-presenting cells. J. Immunol. 162, 1723–1729 (1999).
Hagenbaugh, A. et al. Altered immune responses in interleukin 10 transgenic mice. J. Exp. Med. 185, 2101–2110 (1997).
Fiorentino, D.F., Bond, M.W. & Mosmann, T.R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J. Exp. Med. 170, 2081–2095 (1989).
Mosmann, T.R., Cherwinski, H., Bond, M.W., Giedlin, M.A. & Coffman, R.L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136, 2348–2357 (1986).
Lal, C.S. et al. Hypocholesterolemia and increased triglyceride in pediatric visceral leishmaniasis. Clin. Chim. Acta 382, 151–153 (2007).
Shrivastava, S. & Chattopadhyay, A. Influence of cholesterol and ergosterol on membrane dynamics using fluorescent probes. Biochem. Biophys. Res. Commun. 356, 705–710 (2007).
Ni, C.Z. et al. Molecular basis for CD40 signaling mediated by TRAF3. Proc. Natl. Acad. Sci. USA. 97, 10395–10399 (2000).
London, E. & Brown, D.A. Insolubility of lipids in Triton X-100: physical origin and relationship to sphingolipid/cholesterol membrane domains (rafts). Biochim. Biophys. Acta 1508, 182–195 (2000).
Hostager, B.S. et al. Recruitment of CD40 and tumor necrosis factor receptor-associated factors 2 and 3 to membrane microdomains during CD40 signaling. J. Biol. Chem. 275, 15392–15398 (2000).
Pei, Y. & Tuschl, T. On the art of identifying effective and specific siRNAs. Nat. Methods 3, 670–676 (2006).
Nishitsuji, H. et al. Expression of small hairpin RNA by lentivirus-based vector confers efficient and stable gene-suppression of HIV-1 on human cells including primary non-dividing cells. Microbes Infect. 6, 76–85 (2004).
Santhosh, C.V., Tamhane, M.C., Kamat, R.H., Patel, V.V. & Mukhopadhyaya, R. A Lentiviral vector with novel multiple cloning sites: stable transgene expression in vitro and in vivo. Biochem. Biophys. Res. Commun. 371, 546–550 (2008).
Gupta, S., Boppana, R., Mishra, G.C., Saha, B. & Mitra, D. HIV-1 Tat suppresses gp120-specific T cell response in IL-10-dependent manner. J. Immunol. 180, 79–88 (2008).
Acknowledgements
Anti-CD40 was from G. Klaus (National Institute of Medical Research). Supported by the Department of Biotechnology, New Delhi; the Government of India; Centre Franco-Indien pour la Promotion de la Recherche Avancée, New Delhi; and the Council of Scientific and Industrial Research, New Delhi (A.R.).
Author information
Authors and Affiliations
Contributions
A.R. and R.D., all fractionation studies; A.R. and M.J., in vivo experiments; R.K. and S.C., shRNA vector and virus preparation; and S.M., R.M. and B.S., manuscript preparation.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Supplementary Tables 1–2 (PDF 771 kb)
Rights and permissions
About this article
Cite this article
Rub, A., Dey, R., Jadhav, M. et al. Cholesterol depletion associated with Leishmania major infection alters macrophage CD40 signalosome composition and effector function. Nat Immunol 10, 273–280 (2009). https://doi.org/10.1038/ni.1705
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1705